This set of Biophysics Multiple Choice Questions & Answers (MCQs) focuses on “Partition Chromatography – Set 2”.
1. This picture shows a result of Liquid – Liquid chromatography where an immiscible liquid stationary phase is embedded over solid support. Which sample is the highly soluble liquid stationary phase and which one is the least?
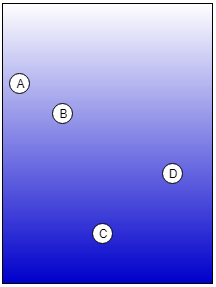
a) Sample C is the highly soluble and Sample B is the least in liquid stationary phase
b) Sample C is the highly soluble and Sample D is the least in liquid stationary phase
c) Sample A is the highly soluble and Sample B is the least in liquid stationary phase
d) Sample C is the highly soluble and Sample A is the least in liquid stationary phase
View Answer
Explanation: If the sample is highly soluble in the stationary phase, it will travel the least distance with the mobile phase due to interaction with the stationary phase and vice versa. Therefore, Sample C is the highly soluble and Sample A is the least in the liquid stationary phase.
2. Only volatile compounds (without decomposition) can be separated using HPLC technique.
a) True
b) False
View Answer
Explanation: In HPLC technique the compounds are separated on the basis of the difference in their boiling point difference or vapor pressure difference. Therefore the sample must be a volatile sample to be eligible for this experiment.
3. Imagine a planner Liquid – Liquid chromatography where an immiscible liquid stationary phase is embedded over solid support. The samples are separated on the basis of their partition coefficient. If the distance traveled by a compound is 8cm and distance traveled by Solvent is 12 cm, then what is the Rf value for that particular solute?
a) 0.6667
b) 1.5
c) 1
d) 2
View Answer
Explanation: Rf value = (Distance traveled by the solute molecule from the origin/ Distance traveled by the solvent front from the origin of samples). So the Rf value is 8/12 = 0.6667.
4. A solute has a partition coefficient 0.65 in a partition chromatography experiment involving two immiscible liquids. What can you infer from this value?
a) The solute is more soluble in upper phase liquid
b) The solute is more soluble in lower phase liquid
c) The solute is more soluble in middle phase liquid
d) The solute is not soluble in lower phase liquid
View Answer
Explanation: Partition coefficient K = the sample concentration in the upper phase / the sample concentration in the lower phase. Here K < 1 which means the solute is more soluble in the upper phase than the lower phase (K < 1).
5. A solute is more soluble in the upper liquid phase in a partition chromatography experiment involving two immiscible liquids. What can you infer from this phenomenon?
a) Partition coefficient 1
b) Partition coefficient < 1
c) Partition coefficient > 1
d) Partition coefficient < 0
View Answer
Explanation: Partition coefficient K = the ratio between the sample concentration in the upper phase and the sample concentration in the lower phase. Here the solute is more soluble in the upper liquid phase therefore, the partition coefficient is > 1.
6. In a Gas-Liquid chromatography, what will be the mobile phase?
a) Liquid
b) Gas
c) Solid
d) Semi-solid
View Answer
Explanation: As the name suggests, in a Gas-Liquid chromatography, the mobile phase will be a gas. The stationary phase is the Solid in a Gas-Liquid Chromatography.
7. In which of the following technique is based on the boiling temperature or vapor pressure of the sample?
a) Gas-gas chromatography
b) Gas-Solid chromatography
c) Solid-Liquid chromatography
d) Gas-Liquid Chromatography
View Answer
Explanation: Among the above options, a Gas-Liquid chromatography is based on the boiling temperature or vapor pressure of the sample. HPLC is based on this concept where the compounds are separated on the basis of the difference in their boiling point difference.
8. Which of the following can be used as a mobile phase in a Gas-Liquid chromatography?
a) He
b) O2
c) Cl2
d) Br2
View Answer
Explanation: In a Gas-Liquid chromatography, always an inert gas is used as a mobile phase. This is important so that the mobile phase does not interact with the sample. Among the above options, He is the only inert gas.
9. ______________ is widely used as a mobile phase in Gas-Liquid Chromatography.
a) Al
b) O2
c) N
d) Cl2
View Answer
Explanation: In a Gas-Liquid chromatography, an inert gas is always used as a mobile phase. This is performed so that the sample is separated only on the difference in their vaporizing point.
10. What is the technique demonstrated by the following image?
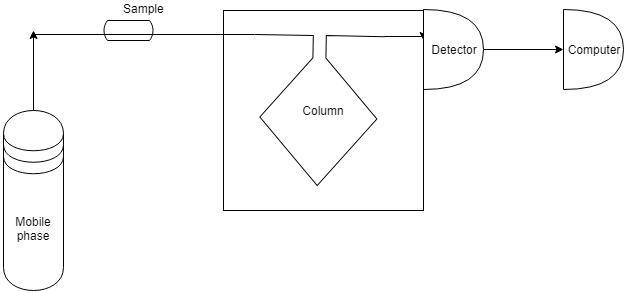
a) Gas–Gas chromatography
b) Gas-Solid chromatography
c) Gas-Liquid chromatography
d) Solid-Liquid chromatography
View Answer
Explanation: The above picture is the demonstration of HPLC (High-pressure liquid chromatography). So it is a Gas-Liquid Chromatography.
11. Who invented partition chromatography?
a) Richard Laurence Millington Synge
b) Newton
c) Archer John Porter Martin
d) Einstein
View Answer
Explanation: Richard Laurence Millington Synge first invented the technique partition chromatography. Archer John Porter Martin first introduced the idea of paper chromatography.
12. Which of the following partition chromatography can never be possible?
a) Liquid – Liquid chromatography
b) Gas – Liquid Chromatography
c) Gas – Gas chromatography
d) Gas-Solid chromatography
View Answer
Explanation: Gas-Solid chromatography cannot be performed as a partition chromatography. This is because partition is a phenomenon of liquid that happens only in liquids and gases.
13. Two volatile samples – X and Y have boiling points 59°C and 69°C respectively. What is the expected result of HPLC?
a) Y will be detected first after that X will be detected
b) X will be detected first after that Y will be detected
c) X will be detected first but Y will not be detected
d) X and Y both will not be detected
View Answer
Explanation: In HPLC, the compounds are separated on the basis of the difference in their vaporizing point difference. Moreover, in HPLC the temperature is increased from lower to higher for separation. So, X as it has a lower boiling point, so it will come first then Y will be detected as it has a higher boiling temperature.
14. From a leaf sample, you have extracted 3 samples two is volatile and another is nonvolatile. How will you separate them?
a) I will simply perform HPLC
b) I will do paper chromatography
c) I will chemically modify another sample into a volatile sample then perform HPLC
d) I will perform partition chromatography
View Answer
Explanation: Only volatile samples can be separated by HPLC so another sample must be modified to a volatile sample so that it can be separated. If it is not done, then the nonvolatile sample will block the HPLC tube which is very costly.
15. If the partition coefficient of a sample is equal to 1. What do you expect its solubility in between two immiscible liquid sample?
a) The concentration of the sample will be higher in the upper phase than the lower phase
b) The concentration of the sample will be the same in both the liquids
c) The concentration of the sample will be higher in the lower phase than the upper phase
d) There will be no sample in the upper phase
View Answer
Explanation: Partition coefficient K = the ratio between the sample concentration in the upper phase and the sample concentration in the lower phase. Now if K = 1 the sample concentration will be the same In both the phases.
Sanfoundry Global Education & Learning Series – Biophysics.
To practice all areas of Biophysics, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
