This set of Biophysics Multiple Choice Questions & Answers (MCQs) focuses on “Paper Chromatography”.
1. A coating of stationary phase is made in the case of paper chromatography.
a) True
b) False
View Answer
Explanation: No coating is made as a stationary phase in the paper chromatography. A simple paper is used as a stationary phase. Depending on the requirement, the paper can be composed of a polar or a nonpolar substance.
2. Which of the following is used a stationary phase in paper chromatography?
a) Silica gel
b) Alumina
c) Cellulose
d) Zirconium Oxide
View Answer
Explanation: No stationary phase is used for a coating in paper chromatography. Now, Silica gel, Alumina and Zirconium Oxide is used as a coating material in case of Thin Layer Chromatography. Only cellulose is used for a coating in paper chromatography.
3. In a particular type of paper chromatography, the mobile phase is allowed to run for two times. What is the name of that type of paper chromatography?
a) Two-dimensional chromatography
b) Horizontal chromatography
c) Ascending chromatography
d) Descending chromatography
View Answer
Explanation: In two-dimensional paper chromatography, the mobile phase is allowed to run for two times. The sample is spotted on the corner then allowed to run the mobile phase. Again the mobile phase is run at a 90° angle for better resolution.
4. In ____________ paper chromatography, sample travels from the center to the periphery of the paper.
a) two dimensional
b) radial
c) ascending
d) descending
View Answer
Explanation: In paper radial chromatography, the paper is circular in nature. The sample is loaded in the center and it travels from the center to the periphery of the paper forming a circular zone of sample spots.
5. What type of chromatography is described in the following picture?
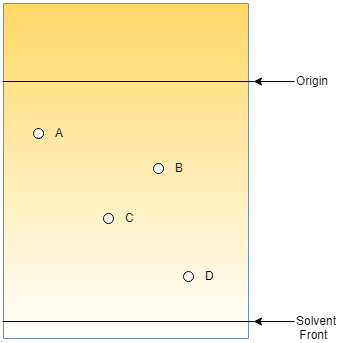
a) Ascending – Descending
b) Radial
c) Ascending
d) Descending
View Answer
Explanation: The descending chromatography is a type of chromatography where the sample is moved from top to bottom. Here, the sample is separated by capillary action along with gravitational force.
6. What is the color of the final complex with alpha-amino acid and Ninhydrin reaction?
a) Blue
b) Orange
c) Red
d) Black
View Answer
Explanation: The overall reaction for the Ninhydrin reaction is α – amino acid + Ninhydrin = Aldehyde + Blue colored final complex+ H2O + CO2. So the final product is blue for α – amino acid.
7. Paper chromatography separates molecules based on which property?
a) Molecular weight
b) Polarity
c) Viscosity
d) Shape
View Answer
Explanation: Paper chromatography separates molecules based on its polarity. The concept is the more it’s attracted to the stationary phase the less it is travel in the paper chromatography.
8. You have to do an experiment with 4 amino acids and all are nonpolar. The amino acids are Ile, Gly, Val, Ala. Arrange the following according to their increasing distance traveled in standard paper chromatography.
a) Ala < Val < Ile < Gly
b) Val < Ile < Gly < Ala
c) Gly < Ile < Ala < Ile
d) Gly < Ala < Val < Ile
View Answer
Explanation: If you look into the structure of each amino acids, the length of the hydrophobic chain increases in the order Gly < Ala < Val < Ile. So the order of nonpolarity is Gly < Ala < Val < Ile. Now, in a standard paper chromatography nonpolar solvent is used as a mobile phase. So the most nonpolar compound will move the highest distance. Therefore, the order of their increasing distance traveled in standard paper chromatography is Gly < Ala < Val < Ile.
9. You had a mixture of monosaccharides. To develop the paper chromatogram, what will you use?
a) Alkaline tetrazolium
b) KMnO4
c) Silica
d) Ninhydrin
View Answer
Explanation: KMnO4 is an oxidative agent, so it will reduce the sugar and develop the spot. KMnO4 will be used to develop the paper chromatogram of a mixture of monosaccharides.
10. Which one of the following has the highest Rf value compared to other options?
a) Arg
b) Cys
c) Thr
d) Val
View Answer
Explanation: Among the following amino acids valine is the only nonpolar amino acid. The rest of the amino acids are polar. Therefore, valine will travel the highest distance in paper chromatography, so it will have the highest Rf value.
11. Which one of the following has the least Rf value compared to other options? (Amino acids are mentioned in their respective one letter code)
a) K
b) E
c) V
d) I
View Answer
Explanation: K, V and I all are nonpolar amino acids so they will have high Rf value. Whereas, E or glutamic acid is a polar amino acid, so it will have the least Rf value.
12. What will you use to develop a chromatogram of cortisol compounds?
a) Alkaline tetrazolium
b) KMnO4
c) Silica
d) Ninhydrin
View Answer
Explanation: Ninhydrin is used to develop a chromatogram of amino acids. KMnO4 is used to develop a chromatogram of monosaccharides. Alkaline tetrazolium reacts with cortisol compounds so it’s used to develop a chromatogram of cortisol compounds.
13. Which of the amino acid show exception chromatogram with Ninhydrin reaction?
a) Arginine
b) Cystine
c) Proline
d) Alanine
View Answer
Explanation: Proline shows exception chromatogram with Ninhydrin reaction. Proline after reacting with Ninhydrin reagent produces yellow color whereas other amino acids give bluish color.
14. The following image is a conceptual diagram of ascending paper chromatography. The paper used in this experiment was made of nonpolar constituents. If the solvent used in this experiment is highly polar then arrange the following compounds according to their increasing order of polarity.
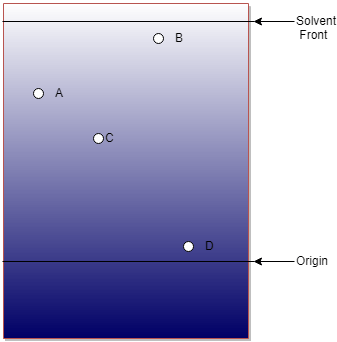
a) A<D<C<B
b) D<C<B<A
c) A<B<C<D
d) D<C<A<B
View Answer
Explanation: The paper used in this experiment was made of nonpolar constituents and the solvent used in this experiment is highly polar; both favors the mobility if a polar compound. So grater mobility means more polar the compound is. Therefore, the correct order is D<C<A<B.
15. In descending paper chromatography, what are the forces responsible for the resolution of separation?
a) Capillary force
b) Capillary force + gravitational force
c) Capillary force + electrostatic force
d) Gravitational force + electrostatic force
View Answer
Explanation: Descending chromatography means sample moves from upper to bottom. So the major forces responsible for the resolution of separation are Capillary force and gravitational force.
Sanfoundry Global Education & Learning Series – Biophysics.
To practice all areas of Biophysics, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
