This set of Solid State Chemistry Multiple Choice Questions & Answers (MCQs) focuses on “Growth of Single Crystals”.
1. The diagram given below represents which of the following method?
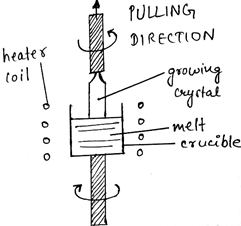
a) Bridgman method
b) Stockbarger method
c) Czochralski method
d) Zone melting method
View Answer
Explanation: Czochralski method is basically a method for the growth of a single crystal from a melt of the same composition. It is also widely used for the growth of the crystals of semiconducting materials, Si, Ge, GaAs, etc. It has also been used to produce laser generator materials such as Ca(NbO3)2 doped with neodymium.
2. In the process of Czochralski method which of the following relation is appropriate between the melt and the growing crystals?
a) Melt and the growing crystals are usually not related to each other
b) Melt and the growing crystals are usually rotated counterclockwise
c) Melt and the growing crystals are usually rotated clockwise
d) Melt and the growing crystals are usually kept at a constant position
View Answer
Explanation: The melt and the growing crystals are usually rotated counterclockwise during pulling in the process of Czochralski, in order to maintain a constant temperature, melt uniformity, etc.
3. Which of the following statements is appropriate for Stockbarger method?
a) Solidification is achieved by passing the melt through a concentration gradient
b) Solidification is achieved by passing the melt through a temperature gradient
c) Liquefaction is achieved by passing the melt through a concentration gradient
d) Liquefaction is achieved by passing the melt through a temperature gradient
View Answer
Explanation: Stockbarger method is based on solidification of stoichiometric melt but in these, oriented solidification of the melt is achieved by effectively passing the melt through a temperature gradient such that crystallization occurs at the cooler end. Thus this method is achieved by arranging for a relative displacement of the melt and a temperature gradient.
4. Which of the following statements describes best the Bridgman method?
a) Melt is outside the temperature furnace, solidification begins at the hotter end
b) Melt is inside the temperature furnace, solidification occurs at the cooler end
c) Melt is inside the temperature furnace, solidification occurs at the hotter end
d) Solidification is achieved by passing the melt through a temperature gradient
View Answer
Explanation: In the Bridgman method, the melt is inside a temperature gradient furnace and the furnace is gradually cooled so that the solidification begins at the cooler end. In this method, it is again advantageous to use a seed crystal and atmospheric control may be necessary.
5. In the zone melting method _____________ of the charge is melted at any one time. Fill up the correct option for the blank space from the choices given below.
a) Large part
b) Small part
c) Solid part
d) Anionic part
View Answer
Explanation: In zone melting method the thermal profile through the furnace is such that only a small part of the charge is melted at any one time. Initially, that part of the charge in contact with the seed crystal is melted. As the boat is pulled through the furnace, oriented solidification onto the seed occurs and at the same time, more of the charge melts.
6. Which one of the following principle has been used in the zone melting method?
a) Impurities concentrate in the solid than in liquid phase
b) Impurities concentrate in the liquid phase than in gaseous phase
c) Impurities concentrate in the liquid phase than in the solid phase
d) Impurities concentrate in the gaseous phase than in the solid phase
View Answer
Explanation: Zone melting method make the use of the principle that impurities usually concentrate in the liquid rather than in the solid phase. Impurities are therefore ‘swept out’ of the crystal by the moving molten zone. The method has been used for the purification and crystal growth of the high melting metals such as tungsten.
7. Which one of the following statements is correct for precipitation method?
a) Growth of the crystal from a solvent of same composition to the crystals
b) Growth of the crystal from the solute of same composition to the crystal
c) Growth of the crystal from the gaseous species of different composition to the crystal
d) Growth of the crystal from a solvent of different composition to the crystals.
View Answer
Explanation: In contrast to the methods like zone melting, Stockbarger etc method in which melts solidify to get crystals that have the same composition as the melt, precipitation methods involves the growth of the crystals from a solvent of different composition to the crystals. The solvent may be one of the constituents of the desired crystal example, crystallization of salt hydrate crystals using water as the solvent.
8. In the precipitation method for the growth of the crystal, the solvent melts are often known as___________
a) Electrolyte
b) α-particle
c) β- particle
d) Fluxes
View Answer
Explanation: In the precipitation method which used for the growth of crystals from the solvent of different composition to the crystals, the solvent melts are often called as fluxes since they effectively reduce the melting point of the crystals by a considerable amount. The method has recently been used to grow crystals of β- and β” alumina solid electrolytes using a borate flux.
9. When was the Verneuil flame fusion method first used?
a) 1207
b) 2016
c) 1503
d) 1904
View Answer
Explanation: The Verneuil flame fusion method was used in 1904 for growing crystals of high melting oxides, including artificial gemstones such as ruby and sapphire.
10. Which of the following method has been used to prepare the single crystals of CaO using plasma torch?
a) Stockbarger method
b) Zone melting method
c) Verneuil flame fusion method
d) Bridgman method
View Answer
Explanation: Verneuil flame fusion method has been recently used to prepare the single crystals of CaO with a melting point equal to 2600◦C by using a plasma torch to melt the CaO powder. The starting material in the form of a fine powder is passed through an oxyhydrogen flame or some other high temperature torch or furnace after melting has taken place, the droplets fall onto the surface of the growing crystals where they solidify.
11. What is the advantage of using Czochralski, Bridgman- Stockbarger and Verneuil method?
a) Gives small crystals
b) High tech apparatus
c) Rapid growth rates
d) Uses plasma torch
View Answer
Explanation: The above methods are melt growth methods which are used for the growth of crystals. Advantages of using these melt growth methods are, t gives large crystals, allows rapid growth rates, and requires very simple apparatus. While the disadvantage can be in the crystal quality which can be poor with inhomogeneities and large defect concentrations.
12. What is the disadvantage of using a solution growth method for the growth of the crystals?
a) Rapid growth rates
b) Simple apparatus
c) Slow growth rates
d) Isothermal conditions
View Answer
Explanation: Solution growth methods like the water crystallization, flux growth, hydrothermal method etc, which are used for the growth of the crystals have the disadvantage that it leads to very slow growth rates, face problems of contamination by container or flux. However, the advantage of using such methods are that it allows isothermal conditions with slow growth rates give quality crystals of low defect concentration.
Sanfoundry Global Education & Learning Series – Solid State Chemistry.
To practice all areas of Solid State Chemistry, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Apply for Biotechnology Internship
- Check Solid State Chemistry Books
- Check Biotechnology Books
- Practice Biotechnology MCQs
