This set of Iron Making Multiple Choice Questions & Answers (MCQs) focuses on “Gas Solid Reaction Equilibria in Blast Furnace Stack”.
1. How much percentage of reduction takes place through indirect reduction?
a) 20%
b) 33.33%
c) 66.66%
d) 100%
View Answer
Explanation: In blast furnace 2/3 of the reduction takes place through indirect reduction in stack region. In the blast furnace 1/3 reduction takes place through direct reduction and remaining through indirect reduction mechanism.
2. Which reaction from the following reactions is direct reduction reaction?
a) 3Fe2O3 + CO = 2Fe3O4 + CO2
b) Fe3O4 + CO = 3FeO + CO2
c) FeO + CO = Fe + CO2
d) Fe2O3 + 15/7C = 2Fe + 9/7CO + 6/7CO2
View Answer
Explanation: In this reaction, reduction is directly taking place from C itself. Whereas reduction from CO is taking place in all other reactions. Direct reduction of iron ore from solid carbon is known as direct reduction reaction.
3. Which of the following is Boudouard reaction?
a) 2CO = CO2 + C
b) FeO + CO = Fe + CO2
c) CaO + CO2= CaCO3
d) FeO + CO = Fe + CO2
View Answer
Explanation: Boudouard reaction is the disproportionation reaction of CO into CO2 & C or its vice-versa.
In this reaction C undergo both oxidation and reduction simultaneously.
4. CO (Carbon monoxide) is a ___________
a) oxidizing & decarburizing agent
b) reducing & carburizing agent
c) oxidizing & carburizing agent
d) reducing & decarburizing agent
View Answer
Explanation: Carbon monoxide is not only a reducing agent but, because of reverse of the Boudouard reaction, it is also a carburizing agent according to: 2CO = [C] + CO2. Carburizing stands for addition of carbon.
5. Direct reduction reactions are _____________
a) endothermic and economical of carbon
b) endothermic and uneconomical of carbon
c) exothermic and economical of carbon
d) exothermic and uneconomical of carbon
View Answer
Explanation: Endothermic reaction as during reaction heat is absorbed and economical as less C is required for reduction of iron. Direct reduction is not harmful in blast furnace as fuel consumed for heat generation is compensated by less C utilization for reduction.
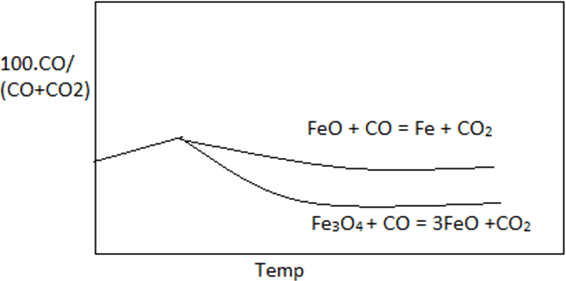
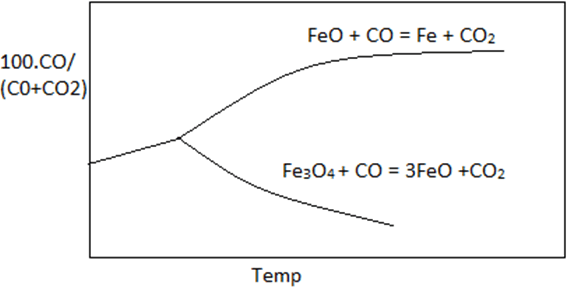
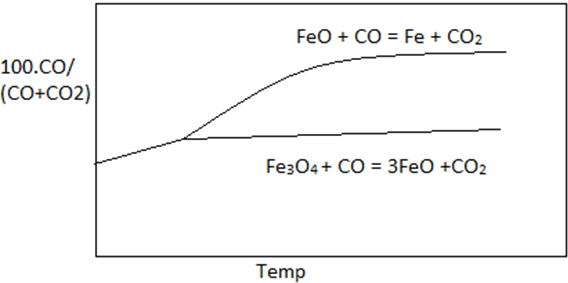
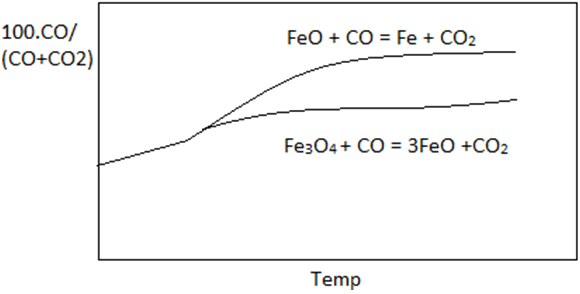
6. Which curve best describe the Fe-O-C equilibrium.
a)
b)
c)
d)
View Answer
Explanation: Graph with maximum difference between the two curves best describe the curve of 100.CO/(CO+CO2) vs temperature. Curve of reaction FeO + CO = Fe + CO2 lies above reaction Fe3O4 + CO = 3FeO + CO2 in the given reference.
7. In FexO (wustite), x lies between __________
a) 0.33-0.66
b) 0.66-0.99
c) 0.835-0.945
d) 0.723-0.903
View Answer
Explanation: Wustite is an oxygen-deficient non-stoichiometric compound. Its general formula is FexO, where 0.835 < x < 0.945. In wustite Fe is present in both +2 & +3 state.
8. Structure of magnetite is __________
a) cubic
b) face centered cubic
c) hexagonal closed-pack
d) body centered cubic
View Answer
Explanation: The chemical formula of magnetite is Fe3O4. It has a cubic structure containing about 27.64% oxygen with a theoretical density of 5.18 g/cm3.
9. Any gas–solid reaction is much faster than a reaction between two solid species.
a) True
b) False
View Answer
Explanation: Diffusion is much higher in solid-gas reaction than in two solid reaction. Reduction of solid iron oxides in a blast furnace occurs through reaction primarily with carbon monoxide in the stack.
10. In a blast furnace, significant decomposition of limestone occurs at _________
a) 1000-1100°C
b) 1100-1200°C
c) 1200-1300°C
d) 1200-1500°C
View Answer
Explanation: In a blast furnace, significant decomposition of limestone occurs at 1000–1100°C. Decomposition of limestone is endothermic and requires heat to be supplied.
11. H2(g) + CO2(g) = H2O(g) + CO(g), reaction is known as _________
a) water gas reaction
b) producer gas reaction
c) H2-CO2 gas reaction
d) CO2 gas reaction
View Answer
Explanation: Reaction is known as the water gas reaction. It is one of the important reactions takes place in the stack. H2O is used to maintain temperature & CO is a reducing agent.
Sanfoundry Global Education & Learning Series – Iron Making.
To practice all areas of Iron Making, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
