This set of Gas Dynamics Multiple Choice Questions & Answers (MCQs) focuses on “First Law of Thermodynamics (Energy Equation)”.
1. Which of the following expression defines the first law of thermodynamics?
a) δQ + δW = dU
b) δQ – δW = dU
c) δQ + δW + dU = 0
d) (δQ / δW) – dU = 0
View Answer
Explanation: The first law of thermodynamics states that: “The energy can neither be created or destroyed, but can only be converted from one form to another.”
Hence,
Total energy added to the system = Energy stored in the system + Total energy leaving the system
i.e. δQ = dU + δW
δQ – δW = dU
2. For an adiabatic process, the internal energy of the system is given by ________
a) ΔU = 0
b) ΔU = ΔQ + ΔW
c) ΔU = – ΔW
d) ΔU = ΔQ
View Answer
Explanation: An adiabatic process refers to the thermodynamic process in which no heat is added or removed from the system i.e. ΔQ = 0. Thus according to the First Law of thermodynamics, the internal energy of the system is given by:
ΔU = ΔQ – ΔW
ΔU = – ΔW
3. Consider the system does the 320 J work when 400 J heat is added to the system, and later there is a heat transfer of 250 J out of the system, while 350 J of work being done on the system. Then what will be the net change of internal energy of the system?
a) 180 J
b) 200 J
c) 150 J
d) 230 J
View Answer
Explanation: Here, net work of the system = Total work is done by the system – Total work done on the system
i.e. ΔW = 320 J – 350 J
ΔW = – 30 J
And, net heat transfer of the system = Total heat added to the system – Total heat removed from the system:
i.e. ΔQ = 400 J – 250 J
ΔQ = 150 J
Therefore as per the first law of thermodynamics, the net change in internal energy of the system:
ΔU = ΔQ – ΔW
ΔU = 150 J – (- 30) J
ΔU = 180 J
4. The process in which both the system and the surroundings are returned to their initial states i.e. the system is in thermodynamic equilibrium with surrounding, at the end of the process, is called ______
a) Adiabatic process
b) Reversible process
c) Irreversible process
d) Isothermal process
View Answer
Explanation: The reversible process is defined as, “The process in which both the system and the surroundings are returned to their initial states i.e. the system is in thermodynamic equilibrium with surrounding, at the end of the process.”
5. For incompressible flow, the energy equation is Bernoulli’s equation.
a) True
b) False
View Answer
Explanation: Since for the incompressible flow, we neglect the change in internal energy and temperature of the system, the energy equation
h + \(\frac {V^2}{2}\) = h0
can be written as
p + \(\frac {\rho V^2}{2}\) = p0 where (h = p / ρ)
Hence for the incompressible flow, the energy equation is the Bernoulli’s equation.
6. If the internal energy of the system is 500 J and the work done by the system is 270 J, calculate the static enthalpy of the system.
a) 250 J
b) 300 J
c) 770 J
d) 230 J
View Answer
Explanation: The enthalpy of the system is given by:
H = U + PV (PV = work done)
H = 500 J – 270 J
H = 230 J
7. The following system refers to the which kind of thermodynamic system?
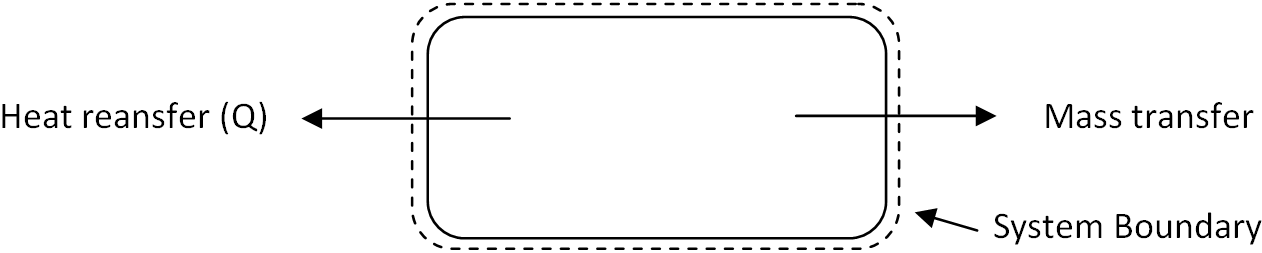
a) Open system
b) Closed system
c) Isolated system
d) Null system
View Answer
Explanation: Since there is both the heat and mass transfer is taking place across the system boundary, it is the open system.
8. Among the followings, in which method the fixed region in space is established with specified control boundaries, and then heat and mass transfer across boundaries are studied?
a) Constant volume approach
b) Constant pressure approach
c) Constant mass approach
d) Constant temperature approach
View Answer
Explanation: Constant volume approach refers to the fixed region being established in space with specified control boundaries, and then heat and mass transfer across boundaries are studied. Whereas in constant mass approach heat and mass transfer are studied across the element as it moves through space.
9. What is the value of change in enthalpy, for an endothermic process?
a) ΔH = + ve or – ve
b) ΔH = – ve
c) ΔH = 0
d) ΔH = + ve
View Answer
Explanation: An endothermic process is a process that absorbs the energy from its surroundings. Thus by definition of enthalpy:
ΔH = U + PV
ΔH = + ve
10. Which process is termed as an adiabatic and reversible process?
a) Isobaric
b) Isochoric
c) Isentropic
d) Isothermal
View Answer
Explanation: The isentropic process is called an adiabatic and reversible process. Whereas an isobaric process refers to a constant pressure process. Isochoric process refers to a constant volume process and isothermal process refers to a constant temperature process.
Sanfoundry Global Education & Learning Series – Gas Dynamics.
To practice all areas of Gas Dynamics, here is complete set of Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
