This set of Extractive Metallurgy Multiple Choice Questions & Answers (MCQs) focuses on “Extraction of Metals from Sulphide Ores – Set 3”.
1. Vertical retort is a ______ process.
a) non-continuous
b) continuous
c) batch
d) cheap
View Answer
Explanation: Vertical retort is a continuous process. The charge is introduced in the retort from the top and heating is provided from the bottom of the chamber. There is the continuous discharging of slag from the bottom of the retort which signifies why it is a continuous process.
2. Which of the following is not a property of copper?
a) High electrical conductivity
b) High resistance to various corrosions
c) Low thermal conductivity
d) Low cost when compared to steel
View Answer
Explanation: Copper is a metal which has unique properties which is difficult to find in any other metal. It has good thermal and electrical conductivities. It has high scrap value. There are many more properties of copper which makes it the most used element in electrical applications.
3. Which of the following is copper ore?
a) Chalcopyrite
b) Rutile
c) Dolomite
d) Corundum
View Answer
Explanation: Chalcopyrite is the most commercially used copper ore. It is a sulphide ore with the chemical formula of CuFeS2. Rutile, Dolomite and Corundum are titanium, magnesium and aluminium ores respectively.
4. Why the grinding process is done during the copper extraction process?
a) To decrease the copper concentration
b) To increase the amount of copper ore
c) To make the addition of Sulphur to copper ore easier
d) To remove the sulphide grains
View Answer
Explanation: The first and foremost step in the copper extraction process is crushing and grinding. Most copper ores contain sulphides in huge concentration. The grinding process is done to remove the sulphide grains from the gangue.
5. Which process is used for the concentration of the copper ore?
a) Oxidation
b) Reduction
c) Froth Floatation
d) Gravity Separation
View Answer
Explanation: Froth floatation is used for the concentration of copper ore after grinding and crushing. Sometimes if the ore contains lead and zinc sulphide too, then differential floatation process is used. In the froth floatation process, xanthate reagent is used as a collector.
6. What compound is used to control the pH in the froth floatation process of copper extraction?
a) SiO2
b) CaO
c) LiF
d) Na
View Answer
Explanation: In the froth floatation process, CaO is added to control the pH of the solution. CaO is an economical reagent for acid neutralization which makes it suitable for the controlling of pH.
7. Why roasting is done in the copper extraction process?
a) To remove Sulphur from the concentrate
b) To remove phosphorus from the concentrate
c) To make the copper extraction process faster
d) To increase the copper content
View Answer
Explanation: The roasting in the copper extraction process is done to remove the Sulphur from the concentrate. Roasting is also done to partially oxidize iron sulphide which is present with copper sulphide. This facilitates its removal further in the smelting operation.
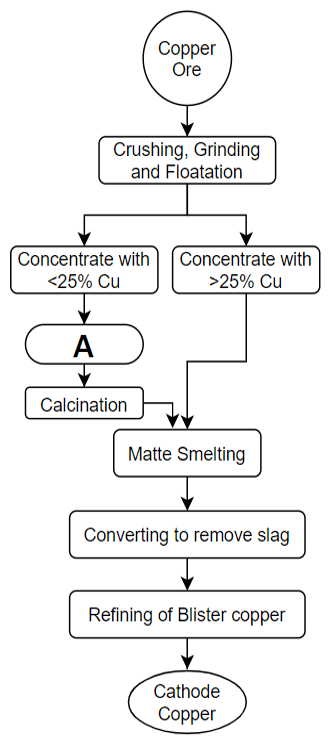
8. What should be there in the box named ‘A’ in the following figure?
a) Sintering
b) Oxidation
c) Reduction
d) Roasting
View Answer
Explanation: The shown flowchart describes the process of copper extraction in brief. ‘A’ is the roasting process in the given flowchart. In copper extraction, the extent of roasting determines the percentage of copper which will we there in the matte after the smelting process. So, concentrates which have copper sulphide content < 25% are passed through the roasting process. Roasting is not necessary for concentrates with copper sulphide content of more than 30%.
9. Roasting process for copper extraction process is done in ______
a) fluidized bed roaster
b) multiple hearth roasters
c) flash roasters
d) chlorinators
View Answer
Explanation: Conventionally, multiple hearth roasters are used for the roasting process of copper ore concentrates. In this type of roaster, the feed is transferred from one hearth to another with the temperature increasing gradually. This roaster is used for the calcination of copper concentrates.
10. What is the purpose of smelting in the copper extraction process?
a) To increase the amount of heat
b) To decrease the concentration of copper
c) To add more copper to the ore
d) To separate metal sulphides in calcine
View Answer
Explanation: After calcination, we obtain calcine which contains not only copper sulphide but also iron sulphide, oxides of iron and some mixed sulphates of copper and iron. The copper and iron sulphides are separated by the smelting process. Smelting also determines the grade of matte.
11. After the smelting process in the copper extraction, two layers of liquid are formed.
a) True
b) False
View Answer
Explanation: The above statement is correct. After the smelting process, two layers are seen. The upper layer consists of gangue and flux. The lower layer is the matte which is the copper and iron sulphides. The density of matte is more than the above layer of slag, so it settles below the slag.
12. What is the advantage of submerged electric arc furnace over reverberatory furnace for smelting process?
a) High energy consumption
b) High thermal efficiency
c) Readily available
d) The high cost of the instrument
View Answer
Explanation: Reverberatory furnace is mostly used for smelting process of copper extraction. The main disadvantage of reverberatory furnace is that the outgoing gases carry away a lot of heat with it. This reduces the thermal efficiency of the furnace. The advantage of using electric smelting is high thermal efficiency and low energy consumption.
13. Converting process in the copper extraction is done to remove copper from the matte.
a) True
b) False
View Answer
Explanation: The statement is not correct. In the copper extraction, after smelting, we obtain a matte. After smelting, converting is done to remove Sulphur, iron and other impurities from the matte. This process is done in a convertor.
Sanfoundry Global Education & Learning Series – Extractive Metallurgy.
To practice all areas of Extractive Metallurgy, here is complete set of Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
