This set of Extractive Metallurgy Multiple Choice Questions & Answers (MCQs) focuses on “Smelting – Set 2”.
1. Which of the following is not a required property of a slag?
a) The difference in specific gravities of slag and metal
b) Slag must be fluid enough for its separation from the metal
c) Slag should be highly viscous
d) Its chemical composition should be such that the activity of impurities is low
View Answer
Explanation: The slag should not be highly viscous. If it is viscous then metal losses are very high due to the difficulty in separation of metal from it. Metal entrapment in the slag will be high. So, this should be avoided.
2. Which furnace can provide a temperature of about 2000°C for a smelting process?
a) Reverberatory furnace
b) Iron Blast furnace
c) Retort
d) Electric arc furnace
View Answer
Explanation: A temperature of around 2000°C can be easily attained in Electric arc furnace. A blast furnace is said to deal with temperatures of around 2000°C but practically carbon fuels cannot help it attain temperature more than 1800°C.
3. Flash smelting is the combination of the processes, ______
a) flash roasting and sinter roasting
b) flash roasting and smelting
c) calcination and smelting
d) roasting and calcination
View Answer
Explanation: Flash roasting and smelting processes combine to form a process called flash smelting. In flash smelting, the concentrate is burned with pure oxygen or preheated air so that a sufficient amount of heat is generated to form the metal and also the slag.
4. Why preheated air or pure oxygen is used during flash smelting?
a) Pure oxygen is easily available
b) Decreases the combustion rate
c) Increases the combustion rate
d) Oxygen is diatomic
View Answer
Explanation: In flash smelting, preheated air or pure oxygen is used to increase the combustion rate. High combustion rate increases the amount of SO2 in the coming outgases. Preheated air also helps to maintain autogenous smelting.
5. In the following diagram of a flash smelter, what does ‘A’ and ‘B’ respectively denote?
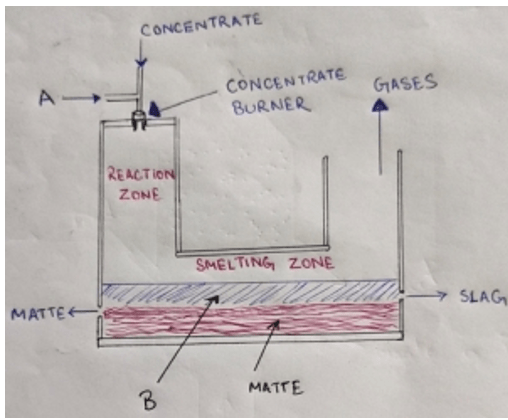
a) Preheated air and slag
b) Carbon dioxide and base metal
c) Preheated air and base metal
d) Gangue and slag
View Answer
Explanation: In Flash smelter, we require a preheated air, so it is passed through the concentrate in a flash smelter. During smelting, matte and slag are separated into two different regions which can be shown in the diagram. Matte settles below slag in the smelting zone.
6. For sulphide ore, matte smelting is what type of process?
a) Hydro
b) Rare metal
c) Electric
d) Thermal
View Answer
Explanation: Matte smelting process for a sulphide ore is a thermally concentrated process. This is because the process takes place at high temperature in a furnace. During this process, Sulphur is lost as gases.
7. A matte is a name for molten sulphate phases which is formed during a smelting process.
a) True
b) False
View Answer
Explanation: The statement is incorrect. A matte is a name for molten sulphide phases which is formed during a matte smelting process. It can contain some minor amount of oxygen and some metal also. It can sometimes be used as a solvent for removal of some impure metals.
8. The electrical conductivity of matte compared to metal is ______
a) high
b) low
c) equal
d) between metal and slag
View Answer
Explanation: When compared to metal, matte has high electrical conductivity. This is because matte removes the impurities which serve as a resistance to electrical conductivities. The density of matte is in between metal and slag.
Sanfoundry Global Education & Learning Series – Extractive Metallurgy.
To practice all areas of Extractive Metallurgy, here is complete set of Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
