This set of Extractive Metallurgy Multiple Choice Questions & Answers (MCQs) focuses on “Distillation”.
1. In the distillation process, one can separate mixtures based on ______ of different components.
a) melting point
b) boiling point
c) molecular formula
d) molecular weight
View Answer
Explanation: In the distillation process, the components of a mixture are separated based on the condition that can change the phase of the components of the mixture. One can separate components based on their boiling points. In this process, there is a conversion of liquid to vapour and then condensed back to its liquid form.
2. The plant where the process of distillation is carried out is known as ______
a) distillery
b) distillationary
c) distil
d) still
View Answer
Explanation: The process of distillation is carried out in a distillery. Normally, alcohols are fermented and processed in a distillery. The process is carried out in an apparatus called still.
3. When is it better to use a selective distillation process?
a) Impurities are less volatile than metal
b) Amount of impurities is more than metal
c) Impurities are more volatile than metal
d) The process is very cheap
View Answer
Explanation: The selective distillation process can be usually applied when the impurities are more volatile than the metals. It lowers the power required for heating. This is not compulsorily applied in every distillation process.
4. It is possible to remove zinc from brass using a selective distillation process.
a) True
b) False
View Answer
Explanation: The statement is correct. Zinc can be selectively distilled from brass due to the very high boiling point of copper. It is usually done at a temperature of about 800°C because above this temperature boiling may become violent.
5. What makes the single-stage distillation a good option?
a) A large difference in the temperatures of the metals to be separated
b) A small difference in the temperatures of the metals to be separated
c) The low difference in the vapour pressure of the metals to be separated
d) The high difference in the vapour pressure of the metals to be separated
View Answer
Explanation: Single-stage distillation can be easily carried out when there is a sufficiently high difference in the vapour pressures of the metals to be separated. This is because, during the distillation process, there is a difference in pressure at different depth levels of the metal.
6. Which of the following technique can be used when the single-stage distillation process cannot produce pure metal concentrates?
a) Rectification
b) Liquation
c) Increasing the temperature
d) Decreasing the temperature
View Answer
Explanation: When the difference in the vapour pressure of the metals to be separated is low then we can use the rectification process to restore pure metals. In this technique, impurities are removed using fractional columns with different temperature distribution.
7. What does the given graph specify?
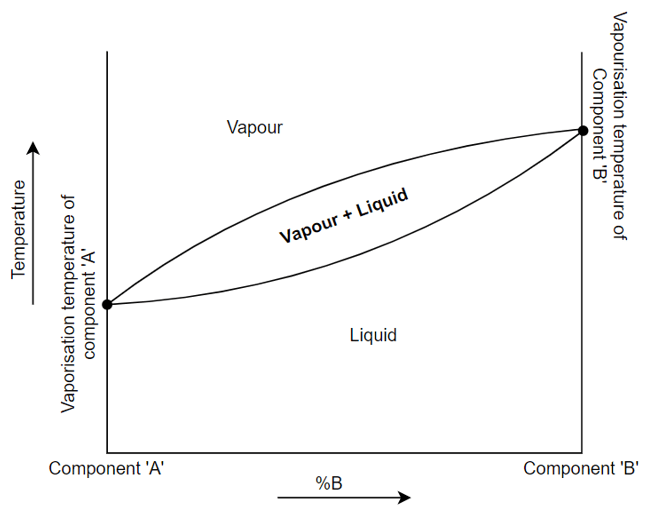
a) Vapour and liquid cannot coexist
b) Vapourization temperature of component ‘A’ is greater than component ‘B’
c) Component ‘A’ vaporizes more easily than component ‘B’
d) At any temperature, vapour has less composition of ‘A’ than liquid
View Answer
Explanation: In the graph shown above, the vapourization temperature of ‘A’ is less than the vaporization temperature of ‘B’. This suggests that component ‘A’ will vapourize easily compared to component ‘B’. From the graph, we can also say that at any temperature, vapour has more composition of ‘A’ than liquid.
8. Which of the following is not a type of distillation?
a) Vacuum
b) Simple
c) Complex
d) Fractional
View Answer
Explanation: Complex distillation is not a type of distillation. Vacuum distillation is the process of distillation done in very low pressure to decrease the boiling point of metals. Simple distillation is the one in which liquid is separated from solid or non-volatile components. In fractional distillation, fractional columns are used in different ranges of temperature.
9. It is impossible to obtain completely pure components after the distillation of a mixture.
a) True
b) False
View Answer
Explanation: The statement is correct. The composition of a constituent in the vapour after the distillation process is dependent on the total vapour pressure of the mixture. The constituents having higher partial pressure has more concentration in vapour. It is impossible to obtain completely pure components after the distillation of a mixture because it is impossible for a component to have zero partial pressure.
Sanfoundry Global Education & Learning Series – Extractive Metallurgy.
To practice all areas of Extractive Metallurgy, here is complete set of Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
