This set of Extractive Metallurgy Multiple Choice Questions & Answers (MCQs) focuses on “Extraction of Metals from Sulphide Ores – Set 2”.
1. What is base bullion?
a) Slag formed after blast furnace operation
b) The lead layer formed after the blast furnace operation
c) Matte formed after blast furnace operation
d) Combination of the layers formed after blast furnace operation
View Answer
Explanation: Base bullion is the name given to the lead layer which is formed after the blast furnace operation. This layer not only contains lead but also contains other valuable elements. This layer consists of lead, copper, silver, gold, arsenic and many other valuable elements. These elements are recovered using various processes.
2. Complete the flowchart of the refining process of lead by filling the question mark area.
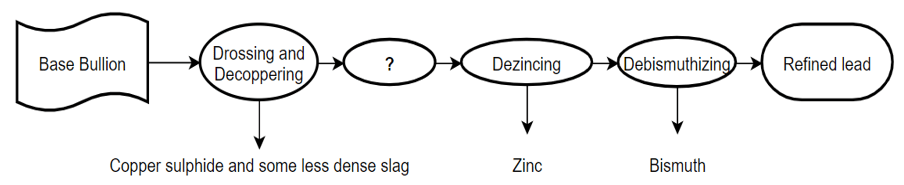
a) Dephosphorization
b) Demagnetizing
c) Kivcet process
d) Parke’s process
View Answer
Explanation: The process which is followed after drossing and decoppering is Parke’s process of desilverization. This process is done for the removal of precious metals like silver from the base bullion. Refining of lead is done to obtain precious and valuable metals which are left in the base bullion.
3. Why zinc is mostly used for galvanization process?
a) Forms a protective layer to resist atmospheric corrosion
b) Zinc is economically affordable
c) Zinc does not react with any compound
d) The atomic mass of zinc satisfy the need for galvanization
View Answer
Explanation: Galvanization is a process in which a protective coating of zinc is applied to steel to protect the material from rusting or atmospheric corrosion. Zinc forms a protective layer of zinc carbonate.
4. Which of the following properties of zinc is not responsible for its varied uses?
a) Low melting point
b) Relatively high cost per unit weight
c) Good structural strength
d) Good formability of metal
View Answer
Explanation: Zinc has many properties for which reason it is used for various purposes like galvanizing, die casting, dry cell batteries and many other uses. Some of its properties are low melting point, low cost per unit weight, good structural strength, good dimensional stability etc.
5. What was the reason for using retort processes for zinc extraction?
a) It produces the highest purity of zinc
b) It uses a hydrometallurgical process
c) It has the highest production rate
d) It solves the condensation problem
View Answer
Explanation: The retort processes solve the condensation problem which is very difficult to tackle. It prevents retort gases to dilute from combustion products. The electrolytic process produces a very high purity of zinc of about 99.95%.
6. Which of the following is not a zinc ore?
a) Sphalerite
b) Calamine
c) Cerussite
d) Smithstone
View Answer
Explanation: Cerrusite is a lead ore with the chemical formula of PbCO3. Sphalerite (ZnS) which is also known as zinc blende is a very common and important ore of zinc. This is always found in galena which is a mineral of lead. Calamine (Zn2O.SiO3) and Smithstone (ZnCO3) are also an ore of zinc but not so common.
7. The process of smelting and converting is not applied to ZnS.
a) True
b) False
View Answer
Explanation: The statement is true. The common process of smelting and converting is not applied to ZnS as in Copper sulphide. This is because the melting point of ZnS is very high. A temperature of around 1500°C is also not satisfactory for this process to melt ZnS.
8. Complete the following reaction which is a part of the pyrometallurgical process of zinc extraction.
ZnS + O2 = ______ + SO2 (The reaction is not balanced).
a) ZnO2
b) ZnO
c) ZnSO3
d) Zn
View Answer
Explanation: The above-given reaction is a part of the pyrometallurgical process for zinc extraction. The reaction takes place during the roasting process. Zinc sulphide is roasted to form zinc oxide which is then processed further for reduction.
9. Why during the pyrometallurgical process of zinc extraction, the roasting is carried out in the fluidized bed roaster?
a) No control over the temperature
b) No other roasting machine is available
c) Very low roasting rate
d) High roasting rate
View Answer
Explanation: During earlier days Hearth roaster was used for the roasting process of zinc concentrate but nowadays, they have been replaced by fluidized bed roaster. There are many reasons for using fluidized bed roaster. Its roasting rate is high. It also has good control over the temperature.
10. Fill the blanks ‘A’ and ‘B’ respectively in the given flowchart.
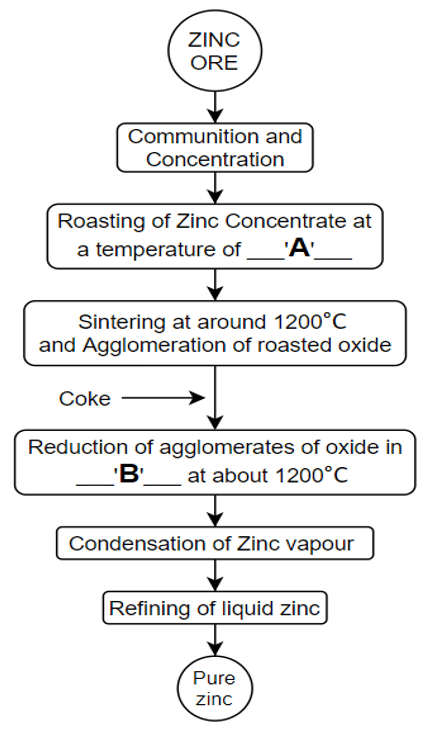
a) 200°C, reverberatory furnace
b) 1800°C, blast furnace
c) 800°C, retort
d) 1500°C, electric arc furnace
View Answer
Explanation: The above-shown flowchart is of the pyrometallurgical process for zinc extraction. In this process, the roasting of zinc concentrate is done at a temperature of about 800°C. After roasting, the zinc oxide is agglomerated. The agglomerated zinc oxide is then transferred to retort for reduction.
11. Why retorts are not made of metal?
a) Most metals are expensive
b) Most of the metals are not reactive
c) Most metals produce zinc alloys
d) Metals produce lots of harmful gases at high temperature
View Answer
Explanation: Retort cannot be made using metals. The reason is that most of the metals alloy with zinc at high temperature. Horizontal Retorts are mostly made of clay. This the reason retorts last for about thirty-five operations.
12. What is the material of which the vertical retorts are usually made of?
a) Silicon carbide
b) Aluminium
c) Iron
d) Titanium
View Answer
Explanation: The vertical retorts are mostly made of silicon carbide. Silicon carbide has a very good conductivity when compared to clay which is the reason silicon carbides are used for vertical retorts.
13. Why the condensation of zinc vapour necessary after the zinc extraction process?
a) Zinc vapour can be stored in a container
b) Zinc vapour can go for a backward reaction
c) The buyers of zinc do not want it in a vapour form
d) Zinc vapour is not economical to store
View Answer
Explanation: In retort, zinc is produced in vapour form. In this form, zinc can be easily oxidized by carbon dioxide. Reversible reactions are possible for high concentrations but for zinc vapour, it can occur for a little concentration of carbon dioxide also.
14. What will happen if the temperature of the condenser, being used for the condensation of zinc vapour, goes below 400°C?
a) Vapour will not condense
b) Vapour will become flammable
c) Zinc will react to form zinc oxide
d) Solid zinc covered with zinc oxide will form excessively
View Answer
Explanation: The temperature of the condenser has to be maintained properly. If the temperature gets too low i.e. below 400°C, then an excess of solid zinc covered with zinc oxide is formed. Also, if the temperature goes very high then the vapour is not condensed properly.
Sanfoundry Global Education & Learning Series – Extractive Metallurgy.
To practice all areas of Extractive Metallurgy, here is complete set of Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
