This set of Heat Treatment of Metals and Alloys Multiple Choice Questions & Answers (MCQs) focuses on “Heat Treatment Principles of Steels”.
1. What is the maximum solubility of carbon in austenite?
a) 2.14%
b) 4.30%
c) 0.022%
d) 0.76%
View Answer
Explanation: The maximum solubility of carbon in austenite is 2.14% and it occurs at 1147°C. In the cast iron, between 723°C and 1147°C, below 4.30% carbon content a mixture of austenite, ledeburite and cementite is present while above 4.30% carbon content a mixture of cementite and ledeburite is present. The maximum solubility of carbon in alpha-ferrite is 0.022%. At 0.76% carbon content, eutectoid steel is found.
2. The formation of austenite from eutectoid steel is different than in the case of hypoeutectoid and hypereutectoid steel.
a) True
b) False
View Answer
Explanation: In case of eutectoid steel, austenite is formed at a particular temperature i.e. 723°C. In case of hypoeutectoid and hypereutectoid steel, transformation takes place over a range of temperature.
3. During formation of austenite, the rate of growth of austenite is higher than the rate of dissolution of cementite into austenite.
a) True
b) False
View Answer
Explanation: The rate of growth of austenite is higher than the rate of dissolution of cementite into austenite. The reason behind this is the transformation of alpha iron to gamma iron and diffusion of carbon atoms from austenite to ferrite. It is this transformation because of which austenitic grain growth takes place.
4. How does the transformation temperature affect the time required for austenite formation?
a) As the temperature increases, the time required for austenite formation increases
b) As the temperature increases, the time required for austenite formation decreases
c) As the temperature increases, the time required for austenite formation may increase or decrease
d) Temperature has no effect on time required for austenite formation
View Answer
Explanation: The austenite formation occurs over a range of temperature which is above eutectoid temperature. As the temperature increases, the rate of transformation increases, thus the time required for austenite formation increases. It can be shown by the diagram below:
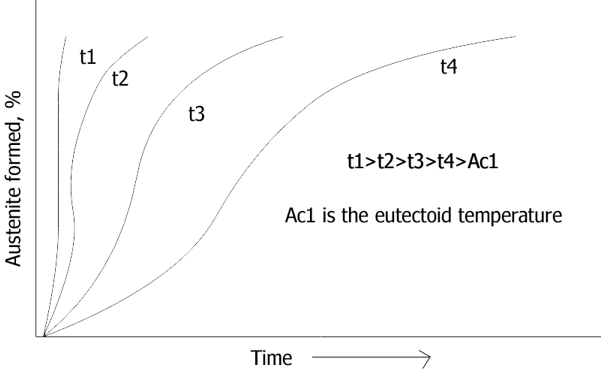
5. High carbon steels austenize at a slower rate than low carbon steels.
a) True
b) False
View Answer
Explanation: The rate of formation of austenite nuclei increases with increases in interfacial area. The interfacial area can be increased by increasing the cementite content which can be done by increasing the carbon content. Thus, high carbon steels austenize more rapidly than low carbon steels.
6. Pearlitic structures with less interlamellar spacing get transformed at a higher rate to austenite.
a) True
b) False
View Answer
Explanation: The number of austenite nuclei formed increases with increase in interfacial area. Interfacial area can be increased by decreasing the interlamellar spacing. Therefore, pearlitic structures with less interlamellar spacing get transformed at a higher rate to austenite.
7. The kinetics for austenitic transformation for coarse pearlite is slower than that for fine pearlite.
a) True
b) False
View Answer
Explanation: In the case of coarse pearlite, there is higher interlamellar spacing. Therefore, the rate of austenite transformation will be lower than that for finer pearlite.
8. The kinetics for austenite transformation of granular pearlite is higher than that for lamellar pearlite.
a) True
b) False
View Answer
Explanation: In the case of lamellar pearlite, the spacing is lesser. Therefore, the carbon atoms have to cover smaller distances by diffusion in order to enrich the lower carbon regions. Therefore, the rate of austenite formation is higher in this case.
9. Quenched structures transform to austenite at a higher rate than granular pearlite.
a) True
b) False
View Answer
Explanation: Quenched structures show high hardness which means that they have fine pearlitic structure. Therefore the interlamellar spacing is less. Thus the carbon atoms need to diffuse to lesser distances which increases the nucleation rate.
10. For alloy steels, how does the kinetics of the formation of austenite depend on carbide forming element?
a) Kinetics in increased in presence of carbide forming elements
b) Kinetics is slower in presence of carbide forming elements
c) Kinetics may increase or decrease in presence of carbide forming elements
d) Carbide forming elements have no effect on the kinetics
View Answer
Explanation: The rate of formation of austenite is decreased in the presence of carbide forming elements. The reason is that the rate of dissolution of alloy carbides in austenite is lesser than that for iron carbides.
Sanfoundry Global Education & Learning Series – Heat Treatment of Metals and Alloys.
To practice all areas of Heat Treatment of Metals and Alloys, here is complete set of Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
