This set of Heat Treatment of Metals and Alloys Multiple Choice Questions & Answers (MCQs) focuses on “Steels – Alloying Elements Effects on TTT Diagram”.
1. Which one of the following elements shifts the TTT curve to right?
a) Boron
b) Aluminium
c) Cobalt
d) Silicon
View Answer
Explanation: Except Aluminium, Cobalt, Silicon, almost all other alloying elements shifts the TTT curve to right. Addition of Boron increases the stability of super cooled austenite thereby increasing the austenite region in the TTT diagram.
2. Which one of the following elements shifts the TTT curve downward?
a) Beryllium
b) Phosphorus
c) Vanadium
d) Nickel
View Answer
Explanation: Nickel stabilizes the austenite. Therefore it shifts the TTT curve downward to lower temperatures. P, V, Ni shift the curve upward as they stabilize the ferrite phase.
3. Which one of the following elements shifts the TTT curve upward?
a) Titanium
b) Manganese
c) Copper
d) Platinum
View Answer
Explanation: Titanium stabilizes the ferrite phase therefore it increases the eutectoid temperature. Thus it shifts the TTT curve upward. Manganese, Copper, Platinum are austenite stabilizers thus they shift the TTT curve downward.
4. Which one of the following elements shifts the TTT curve to the left direction?
a) Nickel
b) Aluminium
c) Copper
d) Gold
View Answer
Explanation: Aluminium shifts the TTT curve to the left. It increases the rate of nucleation and growth of pearlite and ferrite. Nickel, copper and gold are austenite stabilizers.
5. Which one of the following elements encourages the bainitic reaction?
a) Molybdenum
b) Silicon
c) Aluminium
d) Cobalt
View Answer
Explanation: Molybdenum encourages the bainitic reaction. It is added in the sample to prevent grain boundary embrittlement.
6. What is the special characteristic of TTT diagram of high alloy steels?
a) It has resemblance with plain carbon steel
b) It has both pearlitic and bainitic bays
c) It does not have bainitic bay
d) It does not have pearlitic bay
View Answer
Explanation: The TTT diagram of high alloy steels does not have bainitic bay as shown in the figure. Also the Ms temperature shifts to subzero region in this case.
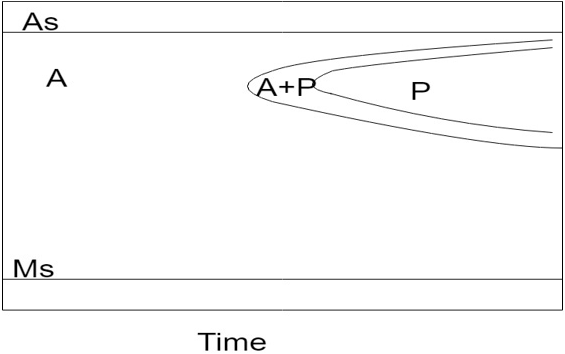
7. Which one of the following statements is correct?
a) Cobalt increases the tendency of decomposition of austenite
b) Alloy carbides have greater stability than cementite
c) Pearlitic transformation involves diffusion of carbon atoms only
d) Bainitic transformation involves diffusion of both carbon and metallic atoms
View Answer
Explanation: Alloy carbides have greater stability than cementite. They decrease the rate of austenite decomposition. Strong carbide formers have a greater effect on retardation of austenite decomposition than weak carbide formers.
Sanfoundry Global Education & Learning Series – Heat Treatment of Metals and Alloys.
To practice all areas of Heat Treatment of Metals and Alloys, here is complete set of Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
