Here are Heat Treatment of Metals and Alloys MCQs (Chapterwise).
1. What is the purpose of annealing in the heat treatment of metals and alloys?
a) To decrease ductility
b) To induce phase transformation
c) To increase hardness
d) To relieve internal stresses
View Answer
Explanation: Annealing is a heat treatment process used to relieve internal stresses, soften the material, and improve its ductility. It involves heating the metal or alloy to a specific temperature and then cooling it slowly to room temperature.
2. Which heat treatment process involves heating steel to a temperature below its critical point and then cooling it in still air?
a) Normalizing
b) Quenching
c) Annealing
d) Tempering
View Answer
Explanation: Normalizing is a heat treatment process used to refine the grain structure of steel by heating it to a temperature below its critical point and then cooling it in still air. This process improves the mechanical properties of the steel, such as strength and toughness, by producing a more uniform grain structure.
3. For alloy steels, how does the kinetics of the formation of austenite depend on carbide forming element?
a) Carbide forming elements have no effect on the kinetics
b) Kinetics may increase or decrease in presence of carbide forming elements
c) Kinetics is slower in presence of carbide forming elements
d) Kinetics in increased in presence of carbide forming elements
View Answer
Explanation: The rate of formation of austenite is decreased in the presence of carbide forming elements. The reason is that the rate of dissolution of alloy carbides in austenite is lesser than that for iron carbides.
4. Which of the phases have FCC structure?
a) Epsilon carbide
b) Martensite
c) Ferrite
d) Austenite
View Answer
Explanation: Ferrite has BCC structure; epsilon carbide has HCP structure and martensite has BCT structure. Only Austenite has an FCC structure.
5. Which one of the following elements neither forms graphite nor carbide?
a) Manganese
b) Iron
c) Silicon
d) Cobalt
View Answer
Explanation: Iron is a carbide forming element. Manganese is also a carbide forming element. Silicon is a graphite forming element. Cobalt is a neutral element. It does not form graphite or carbide.
6. Which of the following statements are correct for inherently fine grained steels?
a) They support the austenitic growth with increasing temperature
b) They resist the austenitic growth with increasing temperature
c) They do not remain fine after 900°c
d) Kinetics of the austenitic growth is very fast
View Answer
Explanation: As the name suggests, the inherently fine grained steels resist the growth of austenite grains with temperature. Therefore as the temperature increases, the austenite grains do not grow initially. The kinetics of growth is very slow. The austenite grains remain fine at temperatures up to 1050°c.
7. Which of the following elements is most effective in increasing the eutectoid temperature?
a) Tungsten
b) Silicon
c) Molybdenum
d) Titanium
View Answer
Explanation: Effect of alloying elements on the increase in eutectoid temperature follows the order
+ Titanium > Molybdenum > Silicon > Tungsten.
8. Calculate the number of grains per square inch in steel at 100X whose ASTM grain size number is 4.
a) 8
b) 16
c) 2
d) 4
View Answer
Explanation: From the formula n=2N-1
Putting N=4, we get n=24-1
Hence n=23=8.
9. Which one of the following statements is true for coarse grained steels?
a) They have greater toughness than fine grained steels
b) They have greater machinability than fine grained steels
c) They have lower hardenability than fine gained steels
d) They have greater grain boundary area
View Answer
Explanation: Because of lesser grain boundaries, coarse grained steels have higher hardenability than fine grained steels. However, they have lower toughness than fine grained steels but higher machinability than fine grained steels. In coarse grained structure during cooling, chip formation occurs which is responsible for machinability.
10. Which one of the following is not the product of the decomposition of austenite?
a) Pearlite
b) Martensite
c) Delta ferrite
d) Bainite
View Answer
Explanation: The various microstructures that are formed as a result of austenite decomposition are Allotriomorphic ferrite, Widmanstatten ferrite, and intragranular ferrite, Cementite, Pearlite, Bainite and Martensite.
11. What is the most popular method of determining the TTT diagram?
a) Density measurement technique
b) Acoustic emission
c) Dilatometry
d) Salt bath technique
View Answer
Explanation: Salt bath techniques are the most popular method of determining the TTT diagram. Apart from this, there are other techniques like Acoustic emission, Density measurement technique, Dilatometry, etc.
12. What is the special characteristic of TTT diagram of high alloy steels?
a) It does not have pearlitic bay
b) It does not have bainitic bay
c) It has both pearlitic and bainitic bays
d) It has resemblance with plain carbon steel
View Answer
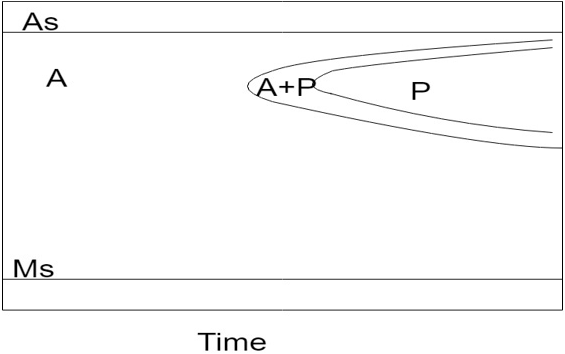
Explanation: The TTT diagram of high alloy steels does not have bainitic bay as shown in the figure. Also the Ms temperature shifts to subzero region in this case.
13. Which one of the following statements is correct regarding ferrite?
a) It has very low carbon content
b) It is pure iron
c) The carbon content vary a lot
d) It has very high carbon content
View Answer
Explanation: Sometimes it is said that ferrite is pure iron but it is not true. Ferrite contains very low carbon content. The carbon content is so low that we assume it to be pure iron for simpler calculations.
14. Which one of the following statements is correct regarding alloy steels which show sharp Bs temperature?
a) They do not have pearlitic bay
b) They do not have bainitic bay
c) They have overlapping pearlitic and bainitic bay
d) They have separate pearlitic and bainitic bay
View Answer
Explanation: Most of the alloy steels do not show sharp Bs temperature. Those steels which show sharp Bs temperature have a separate pearlitic and bainitic bay.
15. What is the structure of the lower bainite?
a) Feathery
b) Plate like
c) Lamellar
d) Accicular
View Answer
Explanation: Lower bainite has accicular structure. Upper bainite has a feathery structure. Pearlite has lamellar structure. Sometimes martensite has plate like microstructure.
16. Which process is involved in martensite formation?
a) Spheroidizing
b) Quenching
c) Normalizing
d) Annealing
View Answer
Explanation: Martensite is formed by quenching austenised steel. It involves rapid cooling without giving any time for diffusion. Quenching results in high hardness values. Brittleness also increases.
17. Plate martensite is formed in which category of steels?
a) High and medium carbon steels
b) Low and high carbon steels
c) High carbon steels
d) Low and medium carbon steels
View Answer
Explanation: Plate martensite is formed in high carbon steels. They are also formed in high alloy steels. Lath martensite is formed in low and medium carbon steels.
18. Which one of the following statements is incorrect regarding annealing?
a) It enhances machinability
b) It refines grain size
c) It reduces chemical non-uniformity
d) It increases the gaseous content in steel
View Answer
Explanation: Annealing process reduces chemical non uniformity. It also refines grain size and enhances machinability. It also reduces the gaseous content in steel thus improving the mechanical properties.
19. What is the final product of full annealing?
a) Coarse pearlite
b) Coarse austenite
c) Fine austenite
d) Fine pearlite
View Answer
Explanation: The main purpose of full annealing is to improve ductility and reduce hardness. Therefore the final product is coarse pearlite. Fine pearlite will lead to the formation of a hard and brittle structure whereas in coarse pearlite, hardness decreases and ductility increases.
20. Which one of the following statements is correct regarding isothermal annealing?
a) It is suitable for both small and heavy components
b) It is suitable for only heavy components
c) It is suitable for only small sized components
d) Any type of steel component can be treated by this process
View Answer
Explanation: One important limitation of isothermal annealing is that it cannot be treated for heavy components. The reason is that it is not possible to cool them rapidly and uniformly at a temperature at which transformation occurs.
21. Which one of the following statements is correct regarding holding time of diffusion annealing?
a) 4-5 hours
b) 5-8 hours
c) 1-2 hours
d) 10-20 hours
View Answer
Explanation: The holding time for diffusion annealing is too long. It is generally 10-20 hours for diffusion annealing. It is then followed by slow cooling.
22. Which one of the following statements is correct regarding partial annealing?
a) Hypereutectoid steel is treated with this
b) Hypoeutectoid steel is treated with this
c) Hypoeutectoid steel and eutectoid steel is treated with this
d) Eutectoid steel is treated with this
View Answer
Explanation: Unlike full annealing and isothermal annealing, which are applicable only for hypoeutectoid steel and eutectoid steel. Partial annealing is used mostly for hypereutectoid steels.
Chapterwise Multiple Choice Questions on Heat Treatment of Metals and Alloys

1. MCQ on Phase Diagrams
The section contains multiple choice questions and answers on phase diagrams and the effect of alloying elements on the Fe-Fe3C phase diagram.
|
|
2. Heat Treatment Principles of Steels
The section covers questions and answers on heat treatment principles of steels, grain size, austenitic grain size determination and importance, decomposition of austenite, transformation curves, effects of alloying elements, transformation types like pearlitic, bainitic, and martensitic, and their kinetics.
3. Heat Treatment Processes for Steels
The section contains MCQs on heat treatment processes for steels, covering stress relieving, annealing including full, isothermal, diffusion, and partial annealing.
|
|
|
Wish you the best in your endeavor to learn and master Heat Treatment of Metals and Alloys!
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
