This set of Powder Metallurgy online test focuses on “Physico-Chemical Methods of Powder Production”.
1. Which process is used for the production of pure reactive metal powder, particularly niobium?
a) Intergranular Corrosion process
b) Oxidation and Decarburization method
c) Gaseous Reduction process
d) Reduction method
View Answer
Explanation: Oxidation and Decarburization method has been developed for the production of pure reactive metal powder, particularly niobium, by reacting metal carbide with metal oxide in vacuum at an elevated temperature so that both oxygen and carbon are removed as CO.
2. Mannesmann process employed for the production of Fe powders can be regarded as oxidation and decarburization method.
a) True
b) False
View Answer
Explanation: Mannesmann process employed for the production of Fe powders (where cast iron is atomized with compressed air) can be regarded as oxidation and decarburization method since it also involves the elimination of both carbon and oxygen.
3. Which of the following method has the principle that grain boundaries are preferential sites for chemical attack than grains?
a) Gaseous Reduction process
b) Hydrometallurgical process
c) Intergranular Corrosion process
d) Oxidation and Decarburization method
View Answer
Explanation: Intergranular Corrosion processis based on the fact that grain boundaries of heat-treated alloys are more susceptible to chemical attack than the grains, thus freeing particles of bulk material.
4. Intergranular Corrosion process was used extensively for the production of which of the following powder?
a) Stainless steel
b) Copper
c) Niobium
d) Brass
View Answer
Explanation: Intergranular Corrosion process was used extensively for the production of stainless-steel (SS) powders. This consists of carburizing SS scrap at a temperature of about 500-750°C for a definite time in order to precipitate chromium carbide at the grain boundaries and corroding the boundary by a boiling aqueous solution of 11% CuSO4 and 10% H2SO4 so as to cause disintegration of the SS into powder.
5. Which process is depicted in the above diagram?
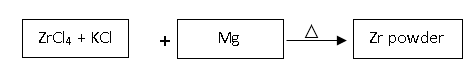
a) Intergranular Corrosion process
b) Precipitation from fused salts method
c) Gaseous Reduction process
d) Precipitation from aqueous solution method
View Answer
Explanation: The process depicted above is precipitation from fused salts, which is generally used for reactive metals. Thus, for the production of Zr powder, the ZrCl4 salt is mixed with an equal amount of KCl and some Mg. Mg replaces the Zr when heated to 749°C and particles of latter settle at the base of the chamber.
6. Which of the following are physicochemical methods of powder production?
a) Thermal decomposition and graining
b) Condensation method and milling
c) Condensation method, gaseous pyrolysis method, and reduction method;
d) Condensation method, gaseous pyrolysis method, reduction method, and cold stream process
View Answer
Explanation: There are 9 physicochemical methods of powder production which are as follows: Condensation method; Gaseous pyrolysis method; Reduction method; Electrodeposition; Precipitation from aqueous solution method; Precipitation from fused salts method; Gaseous Reduction process; Intergranular Corrosion process; and Oxidation and Decarburization method, whereas graining, milling, and cold stream process are mechanical methods of powder production.
7. The process used on a commercial scale for the production of Ni, Co and Cu powder by the hydrometallurgical method is called _____
a) gaseous Reduction process
b) precipitation from fused salts method
c) oxidation and Decarburization method
d) condensation method
View Answer
Explanation: The process used on a commercial scale for the production of Ni, Co and Cu powder by the hydrometallurgical method is called gaseous reduction process where reduction of aqueous solutions or slurries of salts of metals occurs with H2 gas at moderate temperature (130—210°C) and high pressure (400-900 psi).
8. Which of the following is a variant of the hydrometallurgical method?
a) Peace River Process
b) Peace Sea Process
c) Peace Extraction Process
d) Peace Reduction Process
View Answer
Explanation: Peace River Process, has been developed for production of Fe powder with high purity (99.8%), low H2 loss, good pressing properties and sinterability. This process produces a powder with a narrow range of particle size distribution, spherical shape, high apparent densities and flow rates. The process consists of leaching ad Fe- bearing material with HCl, crystallizing the resultant liquor to pure FeCl2, then reducing it to sponge iron followed by grinding it to produce a powder.
9. The method employing the principle of precipitating a metal from its aqueous solution by addition of less noble metal (higher in electromotive series) is called _____
a) condensation method
b) gaseous Reduction process
c) precipitation from aqueous solution method
d) precipitation from fused salts method
View Answer
Explanation: The method employing the principle of precipitating a metal from its aqueous solution by addition of less noble metal which is higher in electromotive series is called precipitation from aqueous solution method. This method produces a very fine metal powder with low apparent density.
10. Which of the following is added to the SnCl2 solution to precipitate Sn?
a) Al
b) Zn
c) Cu
d) Fe
View Answer
Explanation: Tin powder is precipitated by metallic zinc from stannous chloride. Al is used to precipitate Cu/Fe/Ni from their sulphate solution and Cu, Fe is used to precipitate Ag from its nitrate solution.
11. Which of the following is not a characteristic of powder produced by precipitation from an aqueous solution method?
a) Porous in nature
b) High apparent density
c) Low apparent density
d) Difficult to remove the adherent salts
View Answer
Explanation: The precipitated metal powders are generally porous in nature and have low apparent density. A major drawback of the precipitation method lies in the fact that the adherent or entrained salts are more difficult to remove than in electrolytic powder products.
12. Which of the reducing agent is used for the production of Zn powder by condensation method?
a) CaC2
b) Charcoal
c) CO2
d) H2
View Answer
Explanation: Zinc dust (97% pure) is produced by first mixing powdered charcoal to zinc oxide and it is then heated until zinc vapour is formed by the reaction of zinc oxide with carbon monoxide. The Zn vapour is then condensed in a cooler extension of the retort, the condensed vapour is then turned to zinc powder. CaC2 is used for the production of Mg powder.
Sanfoundry Global Education & Learning Series – Powder Metallurgy.
To practice all areas of Powder Metallurgy for online tests, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Practice Metallurgical Engineering MCQs
- Apply for Mechanical Engineering Internship
- Check Mechanical Engineering Books
- Apply for Metallurgical Engineering Internship
- Practice Mechanical Engineering MCQs
