This set of Phase Transformation Multiple Choice Questions & Answers (MCQs) focuses on “Thermodynamics and Phase Diagrams – Binary Solutions”.
1. When X1 mole of A and X2 mole of B are brought together, the corresponding free energy of system is given by___________ (where G1 and G2 are Gibbs energy of pure X1, X2 respectively)
a) X1*G1+X2*G2
b) X1*G2+X2*G1
c) X1*(G1+G2)
d) X2*(G1+G2)
View Answer
Explanation: In order to calculate the free energy of the alloy, the mixing can be made in two steps. After the first step the Gibbs free energy of the system is given by X1*G1+X2*G2.
2. If we consider that there are no volume and enthalpy changes caused by mixing, then the only contribution to the entropy will be__________
a) Thermal
b) Configurational
c) Hydrostatic
d) Rotational
View Answer
Explanation: The only contribution to the entropy will be Configurational. Configurational entropy comes from the possibility of arranging the atoms A and B in different ways for a particular macro state.
3. In an exothermic transformation, the total free energy of the system will change far more drastically with composition at higher temperature compared to a lower temperature.
a) False
b) True
View Answer
Explanation: At higher temperature the entropy term of mixing will be higher, hence Δg of mix will be higher making more drastic changes at higher temperature. Since exothermic transformations involves the release of heat, very high temperatures are expected. As the entropy term –TS depends on the value of T, higher the value of T more will be the change in free energy.
4. In endothermic transformation the enthalpy of mixing is __________
a) Less than zero
b) Zero
c) Greater than zero
d) One
View Answer
Explanation: In endothermic transformation, the enthalpy of mixing is greater than 0. So, if the temperature under consideration is reasonably low, the negative contribution to the Gibbs energy of mixing from entropy term may be smaller than the positive contribution from the enthalpy of mixing within a certain composition range.
5. If we allow inter diffusion to taken place between the elements A and B, there will be change in the free energy because of mixing, if the total molar free energy g of a purely mechanical mixture is 8 Jmol-1
and the ΔG of mix is 5Jmol-1, then the total free energy of the system is ____________
a) 3 Jmol-1
b) 13 Jmol-1
c) 40 Jmol-1
d) 15 Jmol-1
View Answer
Explanation: The total free energy is given by ΔG mix +free energy g. The free energy of the system will not remain constant during the mixing of the A and B atoms and once after mixing free energy of the solid solution G, will depend on the ΔG of mix which comes into picture because of the mixing of the 2 atoms.
6. In case of an ideal system ΔH of mixing is _________
a) Negative
b) Positive
c) Zero
d) Cannot be determined
View Answer
Explanation: In the case of an ideal solution, ΔHof mixing is 0 and the free energy of the system can be written as sum of free energy of mechanical mixture and change in free energy while mixing.
7. The configurational entropy depends on _____
a) Thermodynamic probability
b) Volume
c) Composition
d) Frequency of mixing
View Answer
Explanation: From statistical thermodynamics one can conclude that the configurational entropy depends on the thermodynamic probability, a kind of measure of randomness. Configuration entropy is actually a part of a system’s entropy that is related to distinct representative positions of its constituent particle.
8. Partial molar free energy of a pure substance A or alternatively the chemical potential of A in the phase depends on_____
a) Composition
b) Volume
c) Enthalpy
d) Configuration
View Answer
Explanation: The partial molar free energy or chemical potential of a species in a mixture is defined as the rate of change of free energy of a system (Thermodynamic) with respect to the change in the number of atoms or molecules of the species that are added to the system. If we take the partial derivative into consideration the free energy with respect to the concentration remains constant, hence it depends only on the composition.
9. Assuming that A and B mix to form a substitutional solid solution and that all configurations of A and B atoms are equally probable, the number of distinguishable ways of arranging the atoms on the atom sites, if N1=8 and N2=4 is_____ (N1, N2 are the number of atoms in A and B respectively).
a) 467
b) 499
c) 495
d) 500
View Answer
Explanation: The required equation for the above mentioned problem is (N1+N2)! / ((N1!)*(N2!)). So substituting the value of N1 and N2 we have 12! / (8!*4!) = 495.
10. The variation of activity with composition is given below, out of this which one obeys Raoults law__________
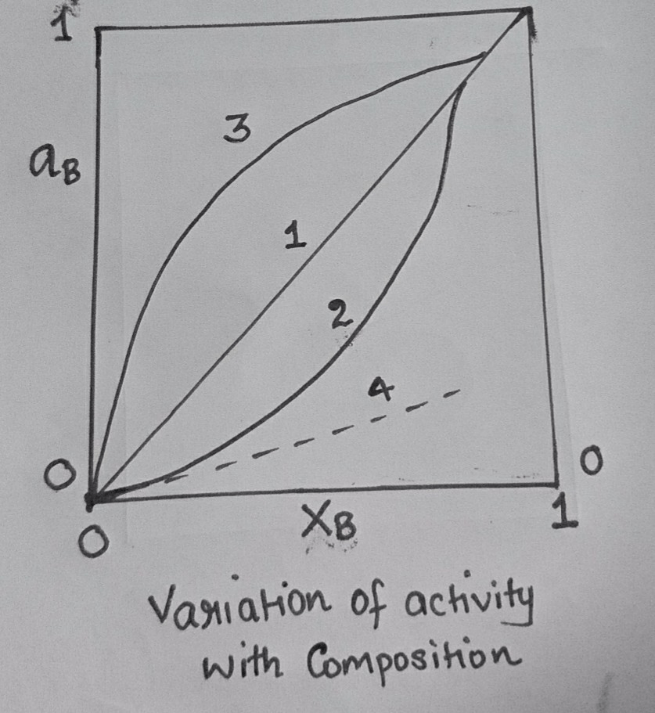
a) 1
b) 2
c) 4
d) 3
View Answer
Explanation: For the Raoults law to satisfy the slope of the line should be 1.Raoults law is a special case of Henrys law. The law takes into account that the vapor pressure of the whole solution will always be less than that of the pure solvent. Therefore, the vapor pressure of the solution will depends on the concentration of the solute.
11. When does the strain energy change can make a difference in the enthalpy of mixing in real solutions?
a) When the temperature is high
b) When the size difference between atoms is very large
c) When the temperature is low
d) When the size difference between atoms is very small
View Answer
Explanation: When the size difference between the atoms is very large then interstitial solid solutions are energetically most favourable and the temperature doesn’t play a big role in making a difference in the enthalpy of mixing in real solutions when the strain energy gets altered.
Sanfoundry Global Education & Learning Series – Phase Transformation.
To practice all areas of Phase Transformation, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Check Metallurgical Engineering Books
- Check Phase Transformation Books
- Apply for Metallurgical Engineering Internship
- Practice Metallurgical Engineering MCQs
- Practice Mechanical Engineering MCQs
