This set of Mechanical Behaviour written test Questions & Answers focuses on “Point Imperfections”.
1. Which one of the following, is not a method of introducing imperfections in metals?
a) Heating
b) Processing
c) Alloying
d) Crystallization
View Answer
Explanation: Heating gives rise to diffusion and thus vacancies. Processing gives rise in dislocations and impurities. Alloying gives substitutional and interstitial defects.
2. Which one is a point defect?
a) Dislocation
b) Vacancy
c) Grain boundary
d) Void
View Answer
Explanation: In a vacancy defect, atom is missing from its normal site in the lattice referred to a point defect. Dislocation is a line and grain boundary is a surface imperfection. Void is a volume imperfection.
3. Which point defect is known as equilibrium defect?
a) Void
b) Dislocation
c) Interstitial
d) Vacancy
View Answer
Explanation: The number of vacancies present in the crystal is in thermal equilibrium and is given by relation n = n0 e(-Q/RT).
Here, n0 is the number of lattice points per cm3.
4. Identify the defect for the below diagram.

a) Frankel
b) Schottky
c) Interstitialcy
d) Vacancy
View Answer
Explanation: Figure shows vacancy-interstitial pair which falls under the category of Frankel defect. Schottky, Interstitialcy and vacancy are absent. The defect in which a metal cation leaves the original lattice position and occupies an interstitial position.
5. Identify the defect for the below diagram.
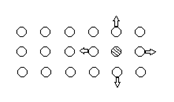
a) Interstitial
b) Substitutional
c) Interstitialcy
d) Schottky
View Answer
Explanation: Figure shows substitutional defect. In this defect parent metal’s atom is substituted by foreign metal atom.
6. Substitutional atoms are generally close in size (approximately 15%) to the parent metal atoms.
a) True
b) False
Viean w Answer
Explanation: Substitutional atom size is close to Parent metal atom. Substitutional atoms substitute in crystal occupying regular lattice position.
7. Which one is not an example of interstitial impurity?
a) Carbon in iron
b) Zinc in brass
c) Hydrogen in Palladium
d) Nitrogen in iron
View Answer
Explanation: Zinc in brass is an example of substitutional impurity. In this, zinc atom (radius 0.133 nm) replaces the copper atom of radius 0.128 nm in brass.
8. If the atom is transferred to an interstitial position instead of the surface, then this defect is known as _____
a) Interstitial
b) Frankel
c) Schottky
d) Vacancy
View Answer
Explanation: When atom transfers to an interstitial position, it is Frankel defect and if the atom transfers to surface, it is known as the vacancy. If impurity atom occupies an interstitial position, it is known as an interstitial defect.
9. In interstitial defect, the atoms surrounding the defect are in the state of tension.
a) True
b) False
View Answer
Explanation: The atom surrounding interstitial defect is in the state of compression because interstitial atoms have sizes larger than the sizes of occupied interstitial sites.
10. For copper, the ratio between interstitial formation enthalpy and the energy required to create a vacancy is _____________
a) 3:1
b) 2:1
c) 1:1
d) 1:2
View Answer
Explanation: Interstitial formation enthalpy is hi~300 kJ/mole (3 eV/atom), which is 3 times greater than the energy required to create a vacancy in copper (~ 100 kJ/mole).
11. Match the following:
i) Oxidation of copper a) Frankel ii) Neutron irradiation causes b) vacancy iii) GaAs (anti site defect) c) interstitialcy iv) Steel (C in Fe) d) interstitial
a) i-b, ii-d, iii-a, iv-c
b) i-b, ii-c, iii-a, iv-d
c) i-c, ii-b, iii-a, iv-d
d) i-d, ii-c, iii-a, iv-b
View Answer
Explanation: Oxidation of copper introduces vacancies while neutron irradiation generates interstitialcy. GaAs generally has Frankel and steel contains interstitial defect.
12. Which alloy system shows complete solubility?
a) Cd-Mg
b) Cu-Zn
c) Fe-Ni
d) Cu-Sn
View Answer
Explanation: Cd-Mg system fulfills all of the four Hume-Rothery rules (atomic size factor, crystal structure rule, vacancy rule and rule) while other systems don’t.
Sanfoundry Global Education & Learning Series – Mechanical Behaviour & Testing of Materials.
To practice all written questions on Mechanical Behaviour, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
