This set of Heat Transfer Operations Quiz focuses on “Dryers – Moisture Isotherms”.
1. The lower the relative humidity _________ is the moisture bound to the host material.
a) Weaker
b) Stronger
c) More quantity
d) Less volume
View Answer
Explanation: The lower the relative humidity, the more strongly is the moisture bound to the host material because the free moisture from the surface has been already lost and the interior layers of moisture are hence strongly bound.
2. The free energy ΔG required to release unit molal quantity of this moisture is given by ___________
a) – RT Ln(Relative Humidity)
b) – RT Ln(Mole fraction of water)
c) – RT Ln(Moisture content)
d) – RT Ln(Absolute Humidity)
View Answer
Explanation: The free energy ΔG required to release unit molal quantity of this moisture is given by the equation, ΔG = – RT Ln(HR) where HR is the relative humidity.
3. What is the term Ψ in the given equation of free energy ΔG required to release unit molal quantity of this moisture?
ΔG = – RT Ln(Ψ)
a) Absolute Humidity
b) Relative Humidity
c) Mole fraction of water
d) Moisture content
View Answer
Explanation: The free energy ΔG required to release unit molal quantity of this moisture is given by the equation, ΔG = – RT Ln(Ψ), where Ψ is the relative humidity. The definition of relative humidity is the ratio of the moisture-vapour pressure to the saturation value at the same temperature.
4. The free energy ΔG required to release unit molal quantity of this moisture is given by
ΔG = – RT Ln(Ψ), for an isothermal, reversible process without change of composition.
a) True
b) False
View Answer
Explanation: Yes, the statement is true. This standard equation of Gibbs free energy can only be applied for an isothermal, reversible process without change of composition.
5. Isothermal variation of the equilibrium moisture content which is a function of this free-energy change with relative humidity yields a ____________
a) Langmuir isotherm
b) Moisture isotherm
c) Adams isotherm
d) Pressure isotherm
View Answer
Explanation: The moisture isotherm is the plot of the relationship between water content and equilibrium relative humidity of a material at equilibrium.
6. Moisture isotherms are normally _________ in shape when plotted over the whole relative humidity range.
a) Sigmoid
b) Exponential
c) Parabolic
d) Hyperbolic
View Answer
Explanation: The sigmoid function represented as below is the correct explanation of the moisture isotherms.
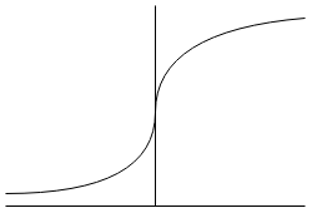
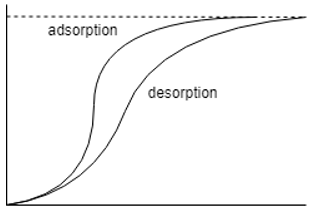
7. Which one of the following isotherm is of prime interest in drying?
a) Langmuir isotherm
b) Desorption isotherm
c) Moisture isotherm
d) Adams’s isotherm
View Answer
Explanation: The desorption isotherm is the diagram of prime interest as it allows us to know how much water has been removed after adsorption and desorption.
8. Consideration of multi-molecular adsorption leads to an equation given below for moisture content.
a) \(\frac{X}{X_1} =\frac{C\phi}{(1-k\phi)(1+(C-k)\phi)}\)
b) \(\frac{X}{X_1} =\frac{C\phi}{(1+k\phi)(1+(C-k)\phi)}\)
c) \(\frac{X}{X_1} =\frac{C\phi}{(1-k\phi)(1-(C-k)\phi)}\)
d) \(\frac{X}{X_1} =\frac{C\phi}{(1-k\phi)(1+(C+k)\phi)}\)
View Answer
Explanation: \(\frac{X}{X_1} =\frac{C\phi}{(1-k\phi)(1+(C-k)\phi)}\) is the required equation for multi-molecular adsorption, the terms are given in the equation are–
- X: it is the moisture content at a relative humidity Ψ which is at equilibrium
- X1: it is the moisture content for a single layer or monolayer
- K: it is the expression e(± ΔH/RT), where ΔH is the enthalpy difference in adsorption heat quantity between the first and successive molecular moisture layers
- C: it is an experimentally calculated coefficient.
9. What is the term X1 in the given equation?
\(\frac{X}{X_1} =\frac{C \phi}{(1-k \phi)(1+(C-k) \phi))}\)
a) Equilibrium moisture content
b) Moisture content for a complete layer
c) Moisture content for three layers together
d) Moisture content for a complete monolayer
View Answer
Explanation: In the equation \(\frac{X}{X_1} =\frac{C \phi}{(1-k \phi)(1+(C-k) \phi))}\) for multi-molecular adsorption, the terms are –
- X: it is the moisture content at a relative humidity Ψ which is at equilibrium
- X1: it is the moisture content for a single layer or monolayer
- K: it is the expression e(± ΔH/RT), where ΔH is the enthalpy difference in adsorption heat quantity between the first and successive molecular moisture layers
- C: it is an experimentally calculated coefficient.
10. What is the term K in the given equation of multi-molecular adsorption?
\(\frac{X}{X_1} =\frac{C\phi}{(1-k\phi)(1+(C-k)\phi)}\)
a) k is the relative humidity
b) k is the exp (± ΔH/RT)
c) k is the moisture content for a complete layer
d) k is the enthalpy difference in adsorptive heats between the first and successive molecular layers
View Answer
Explanation: In the equation \(\frac{X}{X_1} =\frac{C\phi}{(1-k\phi)(1+(C-k)\phi)}\) for multi-molecular adsorption, the terms are –
- X: it is the moisture content at a relative humidity Ψ which is at equilibrium
- X1: it is the moisture content for a single layer or monolayer
- K: it is the expression e(± ΔH/RT), where ΔH is the enthalpy difference in adsorption heat quantity between the first and successive molecular moisture layers
- C: it is an experimentally calculated coefficient.
11. Which one of the following is the correct representation of adsorption desorption diagram?
a) 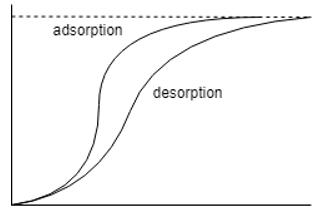
b) 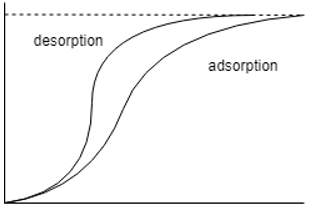
c) 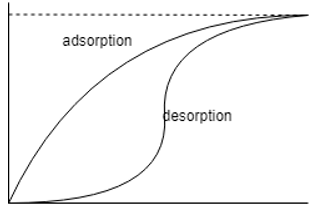
d) 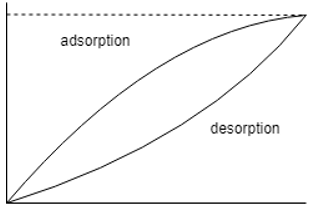
View Answer
Explanation: The moisture isotherms are sigmoid functions and hence the other two options are totally incorrect, the one with the adsorption isotherm above it is the correct option as the desorption isotherm has lesser moisture content than its counter-part.
12. What is the definition of relative humidity?
a) \(\frac{Actual\, vapour\, density}{saturation \, vapour\, density}\)
b) \(\frac{Actual \, vapour \, density}{minimum\, vapour\, density}\)
c) \(\frac{Saturation\, vapour \, density}{maximum \, vapour\, density}\)
d) \(\frac{Actual\, vapour\, density}{Theoretical\, vapour\, density}\)
View Answer
Explanation: The Relative humidity is defined as – the amount of moisture in the air divided by the maximum amount of moisture that could exist in the air at a specific temperature.
Hence the correct formula is \(\frac{Actual\, vapour \, density}{saturation\, vapour\, density}\)
13. What is the name of the axes in the moisture isotherm plotted below?
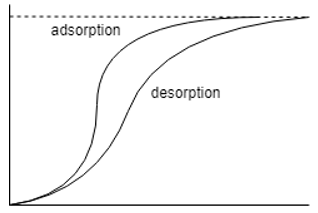
a) X – Relative humidity Y – Moisture content
b) X – Relative humidity Y – Equilibrium moisture content
c) X – Absolute humidity Y – Moisture content
d) X – Absolute humidity Y – Equilibrium moisture content
View Answer
Explanation: The axis of the adsorption desorption moisture isotherms are so set that the adsorption isotherm is less than the desorption ones. Hence the correct axes are X – Relative humidity Y – Equilibrium moisture content.
Sanfoundry Global Education & Learning Series – Heat Transfer Operations.
To practice all areas of Heat Transfer Operations for Quizzes, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Check Heat Transfer Operations Books
- Check Chemical Engineering Books
- Practice Chemical Engineering MCQs
- Apply for Chemical Engineering Internship
