This set of Heat Transfer Operations Multiple Choice Questions & Answers (MCQs) focuses on “Evaporators – Duhring’s Rule”.
1. The Duhring’s rule is used to determine _______
a) Osmotic pressure
b) Surface tension
c) Boiling point elevation
d) Vapour pressure depression
View Answer
Explanation: The Duhring’s rule is used to determine the boiling point of a solution at a particular concentration of solute by assuming that the relation between boiling point solution and solvent is a linear function.
2. The Duhring’s rule states that: The boiling point of a given solution is a _____ function of the boiling point of water at the same temperature.
a) Linear
b) Parabolic
c) Exponential
d) Quadratic
View Answer
Explanation: The Duhring’s rule is used to determine the boiling point of a solution at a particular concentration of solute by assuming that the relation between boiling point solution and solvent is a linear function. It is also necessary to consider that the solution follows Roult’s Law.
3. Identify the correct plot of Duhring’s rule.
a) 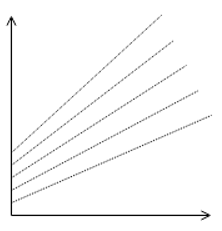
b) 
c) 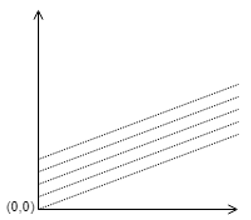
d) 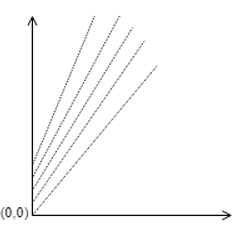
View Answer
Explanation: The plot of Duhring should have slope always greater than 1, and the value of Y-intercept cannot be zero before pure liquid boiling point reaches zero. Hence the most appropriate answer is the given plot.
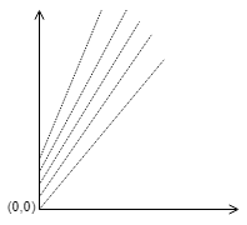
4. What are the names of the axes in the plot by Duhring?
a) X –Concentration of solution, Y- Boiling Point of solution
b) X –Boiling Point of pure water, Y- Boiling Point of solution
c) X –Boiling Point of Solution, Y- Concentration of solution
d) X –Boiling Point of solution, Y- Boiling Point of pure water
View Answer
Explanation: The Duhring’s plot is made between boiling point of pure water as the abscissa and boiling point of solution as the ordinate.
5. For Duhring’s Plot to be valid, the range of boiling points must be relatively ________
a) Narrow
b) Large
c) Very big
d) Negligibly small
View Answer
Explanation: The duhring’s plot should be applied keeping in mind few important concepts of its applications, which is, it cannot be applied for large elevations of BP as well as the solution should follow Roults Law.
6. For Duhring’s plot to be valid and to see its applicability to an operation, we must check if the solution in feed follows __________
a) Henry’s Law
b) Roults law
c) Avogadro’s Law
d) Gas Laws
View Answer
Explanation: The Duhring’s rule is used to determine the boiling point of a solution at a particular concentration of solute by assuming that the relation between boiling point solution and solvent is a linear function. It is also necessary to consider that the solution follows Roult’s Law.
7. The slope of the Duhring’s Plot is always ______
a) Greater than one
b) Greater than 2
c) Less than 1
d) Less than zero
View Answer
Explanation: The Duhring’s plot is done between boiling point of pure water as the abscissa and boiling point of solution as the ordinate. Hence the slope is = B.P.of solution/B.P.of pure water >1 as the B.P.of solution is always greater than B.P.of pure water.
8. If the concentration of the solution increases, the Lines shift __________
a) To the left
b) To the right
c) Downwards
d) Upwards
View Answer
Explanation: The parallel lines of Duhring’s plot are for particular solution concentrations which when increased shifts upwards.
9. Which one of the following is correct?
a) 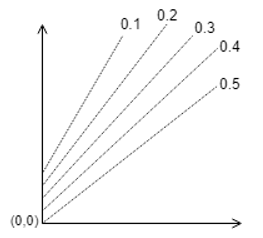
b) 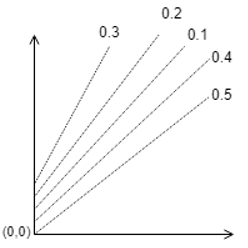
c) 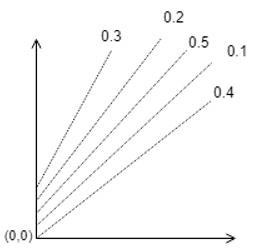
d) 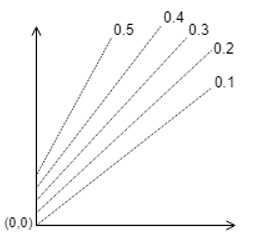
View Answer
Explanation: The parallel lines of Duhring’s plot are for particular solution concentrations which when increased shifts upwards.
10. Determine the Boiling point of solution if the mole fraction is 0.2 and pure water boiling point is 100℃.
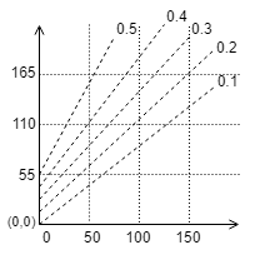
a) 110℃
b) 112℃
c) 100℃
d) 95℃
View Answer
Explanation: We take the point on X-axis as 100 oC, move upwards to intersect the line of mole fraction 0.2, then we extrapolate this to the Y-axis to obtain approximately 112℃.
11. If a = 1min, b = 1hr, c = 2hr, d = 3hr are the operation time of a particular evaporator, then which one of the following plot is correct?
a) 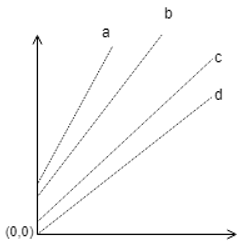
b) 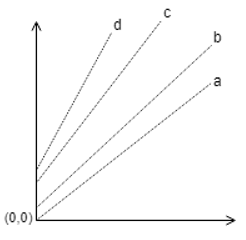
c) 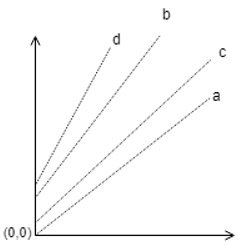
d) 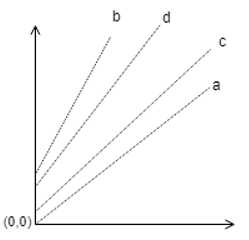
View Answer
Explanation: These lines in the Duhring’s plot correspond to a particular concentration of the solution, hence as the process in the evaporator proceeds, the line moves upwards, hence as time increases, the correct plot is given above.
12. If the boiling point of pure water is 100℃ and the mole fraction is 0.4, then find the boiling point elevation from the given curve.
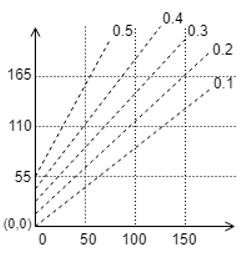
a) 92 ℃
b) 102 ℃
c) 12 ℃
d) 52 ℃
View Answer
Explanation: We take the point on X-axis as 100℃, move upwards to intersect the line of mole fraction 0.4, then we extrapolate this to the Y-axis to obtain approximately 165 + 27 = 192℃, hence the boiling point elevation is = 192-100 = 92℃.
Sanfoundry Global Education & Learning Series – Heat Transfer Operations.
To practice all areas of Heat Transfer Operations, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Apply for Chemical Engineering Internship
- Check Heat Transfer Operations Books
- Practice Chemical Engineering MCQs
- Check Chemical Engineering Books
