This set of Engineering Physics Multiple Choice Questions & Answers (MCQs) focuses on “Zeeman Effect”.
1. Zeeman effect is the splitting of spectral line in the presence of ___________
a) Electric Field
b) Magnetic Field
c) Inert Environment
d) Vacuum
View Answer
Explanation: The splitting of spectral lines into several components in the presence of a static magnetic field is called as Zeeman effect.
2. Zeeman Effect could not be proved by ________
a) Quantum Mechanics
b) Bohr’s Model
c) Hamiltonian operators
d) L-S coupling
View Answer
Explanation: The Bohr’s model could not explain Zeeman effect. It was later by using Quantum Mechanics and the usage of Hamiltonian operators and L-S coupling that it could be explained.
3. The Zeeman effect is not used in ________
a) NMR
b) MRI
c) Optical Amplifiers
d) Laser Cooling
View Answer
Explanation: Zeeman effect is used in NMR spectroscopy, MRI scan and laser cooling. For optical amplifiers, the Raman effect is used.
4. Which of the following is the correct expression for the magnetic moment of the electron?
a) \(\sqrt{n+1}\)
b) \(\sqrt{n(n+1)}\)
c) \(\sqrt{n(n+2)}\)
d) \(\sqrt{m(n+1)}\)
View Answer
Explanation: The magnetic moment of an electron is given by the expression: \(\sqrt{n(n+2)}\). Thus, when the number of unpaired electrons, n, is zero the magnetic moment is zero as well.
5. At-higher magnetic fields, the splitting of spectral lines is disturbed. This effect is called __________
a) Stark Effect
b) Inverse Zeeman Effect
c) Paschen-back effect
d) Anomalous Zeeman effect
View Answer
Explanation: The Paschen–Back effect is the splitting of atomic energy levels in the presence of a strong magnetic field. This occurs when an external magnetic field is sufficiently strong to disrupt the coupling between orbital and spin angular momenta.
6. The perturbation due to the magnetic field is given by ________
a) µ.B
b) µ.E
c) -µ. E
d) -µ. B
View Answer
Explanation: The perturbation on the atom due to the magnetic field is given by -µ. B, where μ is the magnetic moment of the atom.
7. Zeeman effect is used to study which property of sun?
a) Solar flares
b) Sun spots
c) Magnetic fields
d) Electric fields
View Answer
Explanation: George Hale discovered Zeeman effect in the solar spectra, indicating the presence of strong magnetic fields in sun. It is now used to study the magnetic field of sun.
8. Zeeman energy is which energy of a magnetized body?
a) Magnetic Energy
b) Kinetic Energy
c) Potential Energy
d) Total Energy
View Answer
Explanation: The potential energy of a body in a magnetized external field is called the Zeeman energy. It is generated as a result of the interaction between the internal magnetic field of the body and the external magnetic field.
9. The number of split levels in the magnetic field is ________
a) 2n
b) 2n + 1
c) 2l
d) 2l + 1
View Answer
Explanation: The pattern and amount of splitting of spectral lines is a signature of the strength of the magnetic field. The number of split levels are given by 2l + 1.
10. The number of splitting levels of in 2p orbital would be ________
a) 1
b) 2
c) 3
d) 4
View Answer
Explanation: As we know, the splitting levels in an orbital is equal to 2L + 1, where l is the azimuthal quantum number. For a p-orbital, l = 1. Therefore, splitting = 3.
11. In which orbital does the splitting occur?
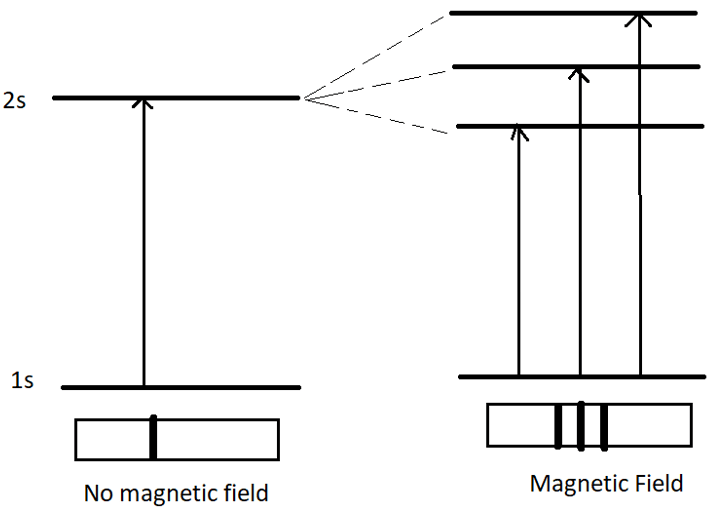
a) s
b) p
c) d
d) f
View Answer
Explanation: As we can see, three spectral lines are observed in the presence of magnetic field. Hence, splitting must have occurred in p-orbital.
12. The lines corresponding to Zeeman effect exhibit polarization effects.
a) True
b) False
View Answer
Explanation: The condition for the Zeeman effect partially aligns the atoms. Thus, when aligned atoms make a transition between two energy states, the lines observed are polarized. Therefore, the lines corresponding to the Zeeman effect shows polarization effects.
13. The magnetic moment of Fe2+ ion is ________
a) 1.8
b) 2.8
c) 3.8
d) 4.8
View Answer
Explanation: We know, that magnetic moment = \(\sqrt{n(n+2)}\), where n is the number of unpaired electrons. In Fe2+ ion, n = 4. Therefore, magnetic moment = √24 ≈ 4.8
14. Zeeman effect and Stark effect are analogous to each other.
a) True
b) False
View Answer
Explanation: The splitting of spectral lines in magnetic field is called Zeeman effect and the splitting in electric field is called Stark effect. Both these effects are analogous to each other.
15. Which of the following have similar magnetic moment: Fe, Fe2+ and Fe3+?
a) Fe and Fe2+
b) Fe and Fe3+
c) Fe3+ and Fe2+
d) Fe, Fe3+ and Fe2+
View Answer
Explanation: Both Fe and Fe2+ have four unpaired electrons while Fe3+ have five unpaired electrons. Thus, Fe and Fe2+ have same magnetic moment.
Sanfoundry Global Education & Learning Series – Engineering Physics.
To practice all areas of Engineering Physics, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
