This set of Engineering Physics Multiple Choice Questions & Answers (MCQs) focuses on “Types of Polarisation”.
1. The four types of polarization are Electronic Polarization, Ionic Polarization, Space-charge polarization and ______
a) Magnetic Polarization
b) Electric Polarization
c) Orientational Polarization
d) Potential Polarization
View Answer
Explanation: The total polarization in a specimen may comprise of the following four components: Electronic polarization, orientational polarization, ionic polarization and space charge polarization.
2. NaCl falls under which category of polarization?
a) Ionic Polarization
b) Space-charge polarization
c) Dipolar Polarization
d) Electronic Polarization
View Answer
Explanation: NaCl contains ionic bond, due to which it has a longer bong length. It is polarized due to the displacement of ions and hence is called, ionic polarization.
3. Electronic polarizability is dependent of temperature.
a) True
b) False
View Answer
Explanation: When there is a displacement in the center of electrons and nuclei due to the presence of an electric field, it is called as electronic polarization. The ration of the induced dipole to the field is called electronic polarizability which is independent of temperature.
4. In which category of polarization Electric field is used to develop a net dipole moment in dipolar substances?
a) Ionic Polarization
b) Space-charge polarization
c) Orientational Polarization
d) Electronic Polarization
View Answer
Explanation: Dipolar substance possess a permanent dipole moment. However, in the absence of an electric field, they are aligned in a way that they cancel out the net dipole moment. Thus, the orientational polarization is due to the fact that it is because of the orientation of pre-existing dipoles.
5. The dipole is least stable when the angle between the dipole and the field is _____________
a) 0°
b) 45°
c) 90°
d) 180°
View Answer
Explanation: The potential energy of a dipole is given by: -pEcosθ. Thus the dipole would be least stable when potential energy would be maximum. Hence, when θ = 180°, i.e., the dipole is aligned opposite the direction of the field, the dipole would be least stable.
6. Space charge polarization is mostly observed in ____________
a) Uni-phasic material
b) Biphasic material
c) Multiphasic material
d) Crystal
View Answer
Explanation: In multiphasic material, the charges accumulate at the interface of the phases and at the electrodes. The ions diffuse over appreciable distances giving rise to space-charge polarization.
7. Which category of polarizability strongly depends on Temperature and frequency?
a) Ionic Polarization
b) Space-charge polarization
c) Orientational Polarization
d) Electronic Polarization
View Answer
Explanation: Orientational Polarizability exhibits strong dependence on both frequency and temperature while the electronic and ionic components are nearly independent of temperature and frequency.
8. The frequency for electronic polarization lies in which region of the electromagnetic spectrum?
a) Microwave
b) Infrared
c) Visible
d) Ultraviolet
View Answer
Explanation: The frequency for electronic polarization lies in the ultraviolet region. For the space-charge it lies in machine frequency, for the dipolar polarization it lies in the microwave region and for the ionic it lies in the infrared region.
9. Which polarizability is the ionic polarizability?
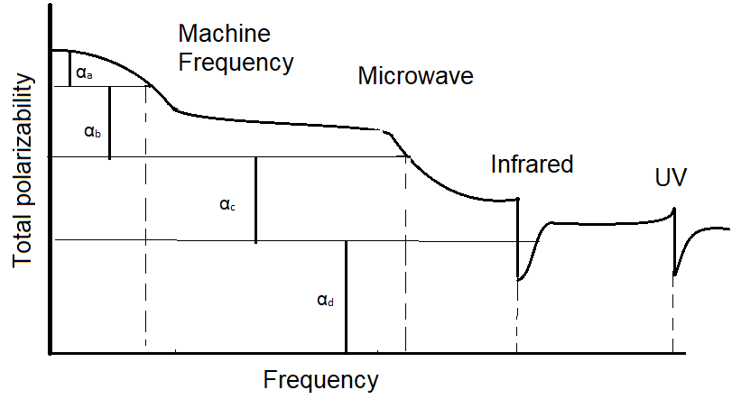
a) αa
b) αb
c) αc
d) αd
View Answer
Explanation: The following graph shows the variation of total polarizability with the frequency. In the graph. αa is for the space-charge polarizability, αb is for the orientational polarizability, αc is the ionic polarizability and αd is the electronic polarizability.
10. What is the polarizability of an argon atom if the relative permittivity of argon at NTP is 1.000435?
a) 1.21 X 10-40 Fm2
b) 1.37 X 10-40 Fm2
c) 1.43 X 10-40 Fm2
d) 1.55 X 10-40 Fm2
View Answer
Explanation: Number of argon atoms at NTP = 6.023 X 1026/22.4
= 2.69 X 1025
We know, εo(εr – 1) = Nαe
αe = εo(εr – 1)/N
= 8.85 X 10-12 X 0.000435/ 2.69 X 1025
= 1.43 X 10-40 Fm2.
11. An oxygen atom produced a dipole moment of 5 X 10-23 cm when subjected to an electric field. If the separation between the center of electronic cloud and the nucleus is 4 X 10-17 m, what is the polarizability of the oxygen atom?
a) 1.4 X 10-47 Fm2
b) 1.7 X 10-47 Fm2
c) 1.8 X 10-47 Fm2
d) 1.9 X 10-47 Fm2
View Answer
Explanation: If d is the separation between the centers, then
E = 8ⅇ/(4πε0 d)
= 8 X 1.6 X 10-19/4 X 3.14 X 8.85 X 10-12 X 16 X 10-34
= 2.6 X 1024 V/m
Also, p = αE
Therefore, α = p/E
= 1.9 X 10-47 Fm2.
12. The dielectric constant of helium is 1.000684. If N is 2.7 X 1025 atoms/m3, what is the radius of the electronic cloud?
a) 3.78 X 10-11 m
b) 4.65 X 10-11 m
c) 5.87 X 10-11 m
d) 6.12 X 10-11 m
View Answer
Explanation: We know, εo(εr – 1) = Nαe
Or, N4πεor3 = εo(εr – 1)
r3 = (εr – 1)/N4π
Here, (εr – 1) = 0.000684, N = 2.7 X 1025 atoms/m3, we get
r3 = 0.000684/4 X 3.14 X 2.7 X 1025
r = 5.87 X 10-11 m.
13. In a water drop of radius 1 mm all the molecular dipole points are in the same direction. If the dipole moment of a water molecule is 6 X 10-30 m, the polarization in the water drop is _____________
a) 6.4 X 10-13 m-2
b) 7.4 X 10-13 m-2
c) 8.4 X 10-13 m-2
d) 9.4 X 10-13 m-2
View Answer
Explanation: Molecular mass of water = 18 gm
18 gm of water contains 6.023 X 1023 molecules
18/103 m3 of water contains 6.023 X 1026/18 molecules
Volume of water drop = 4π/3 X (10-3)3 m3
No of molecules in the drop, N = 6.023 X 1026 X 4π X 10-9/18 X 3
= 1.4 X 1017 m-3
Polarization, P = Np
= 1.4 X 1017 m-3 X 6 X 10-30 m
= 8.4 X 10-13 m-2.
14. The relative dielectric constant of polystyrene is 2.5. What is the polarization produced when 0.5 mm thick sheet of polystyrene is subjected to 220 V?
a) 2.78 X 10-6 C/m
b) 3.91 X 10-6 C/m
c) 4.12 X 10-6 C/m
d) 5.84 X 10-6 C/m
View Answer
Explanation: We know, Polarization, P = εo(εr – 1)E
Here, εr = 2.5
E = V/d
= 220 / 0.5 X 10-3
= 4.4 X 105 Vm
εo = 8.85 X 10-12 C/Vm
Hence, P = 8.85 X 10-12 X (2.5 – 1) X 4.4 X 105 C/m2
= 5.84 X 10-6 C/m.
15. What does the following figure shows?
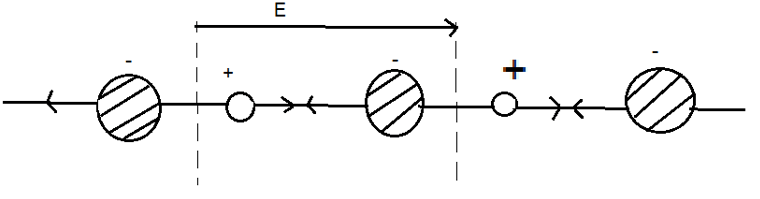
a) Ionic Polarization
b) Space-charge polarization
c) Orientational Polarization
d) Electronic Polarization
View Answer
Explanation: The following figure shows ionic polarization in an ionic crystal. The specimen gets polarized due to the displacement of the ions.
Sanfoundry Global Education & Learning Series – Engineering Physics.
To practice all areas of Engineering Physics, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
