This set of Engineering Physics Multiple Choice Questions & Answers (MCQs) focuses on “Quantum Number”.
1. Quantum Numbers are solutions of _____________
a) Heisenberg’s Uncertainty Principle
b) Einstein’s mass energy relation
c) Schrodinger’s Wave Equation
d) Hamiltonian Operator
View Answer
Explanation: When the wave function for an atom is solved using the Schrodinger Wave Equation, the solutions obtained are called the Quantum Number which are basically n, l and m.
2. Which quantum numbers gives the shell to which the electron belongs?
a) n
b) l
c) m
d) s
View Answer
Explanation: The principal quantum number, n, gives the shell to which the electron belongs. The energy of the shell is dependent on ‘n’.
3. What is the maximum number of electrons in a shell?
a) n
b) 2n
c) n2
d) 2n2
View Answer
Explanation: The total number of electrons in a shell are given by: 2n2 while the number of orbitals present in a shell is given by n2, which is half the total number of electrons in that shell.
4. Which of the following is the correct expression for the orbital angular momentum?
a) \(\sqrt{l+1}\)
b) \(\sqrt{n(l+1)}\)
c) \(\sqrt{l(l+1)}\)
d) \(\sqrt{m(l+1)}\)
View Answer
Explanation: The orbital angular momentum of an electron is given by the expression: \(\sqrt{l(l+1)}\). Thus, when the azimuthal quantum number, l, is zero the angular momentum is zero as well.
5. Which of the following quantum number gives the shape of atomic orbital of sub-shell?
a) n
b) l
c) m
d) s
View Answer
Explanation: The Azimuthal quantum number, l, helps in determining the shape of the atomic orbital of sub-shell. It also gives the sub-shell to which the electron belongs.
6. What is the range of Azimuthal Quantum Number, l?
a) 0 to n
b) 0 to s
c) 0 to n-1
d) 0 to s-1
View Answer
Explanation: The value of Azimuthal Quantum number, l, varies from 0 to n-1. It helps us in identifying to which subshell the electron belongs.
7. The total value of the magnetic quantum number are _______________
a) 2n
b) 2l
c) 2n + 1
d) 2l + 1
View Answer
Explanation: The magnetic quantum number denotes the orientation of electrons in an atom. The total values of me are 2l + 1. They vary from –l to +l.
8. How many values does the spin quantum number have?
a) 2
b) 2l
c) 2n
d) 2me
View Answer
Explanation: The spin quantum number have only two values: +1/2 and -1/2. It is not a solution of the Schrodinger wave equation.
9. Which of the following can be the quantum numbers for an orbital?
a) n = 4, l = 4, m = 3
b) n = 2, l = 3, m = 1
c) n = 3, l = 2, m = -1
d) n = 3, l = 0, m = -3
View Answer
Explanation: In the given options, option c is the correct option because in this the value of l is between 0 – n-1 and value of m is between –l to +l.
10. An electron makes a transition from n = 5 state to n = 2 state in the hydrogen atom. What is the frequency of the emitted photon?
a) 4.9 X 1014 Hz
b) 5.9 X 1014 Hz
c) 6.9 X 1014 Hz
d) 7.9 X 1014 Hz
View Answer
Explanation: The electron makes a transition from n = 5 to n = 2.
ΔE=\(R(\frac{1}{n_1^2}\frac{-1}{n_2^2})\)
= 45.774 X 10-20J
Frequency, v = ΔE/h
= 6.9 X 1014 Hz.
11. What is the azimuthal quantum number for the following sub shell?
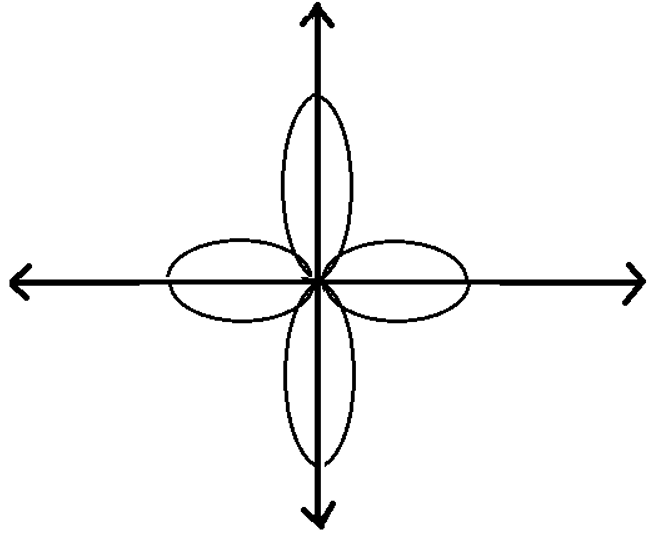
a) 1
b) 2
c) 3
d) 4
View Answer
Explanation: The given figure is the subshell dx2-y2. The azimuthal quantum number for this subshell is 2. It has a total of 5 subshells.
12. The subshell dz2 has no nodal plane.
a) True
b) False
View Answer
Explanation: The subshell dz2 has a ring in x-y plane and is based on the z-plane. Thus the probability of finding an electron is never zero for this sub-shell.
13. The following is the wave function for which orbital?
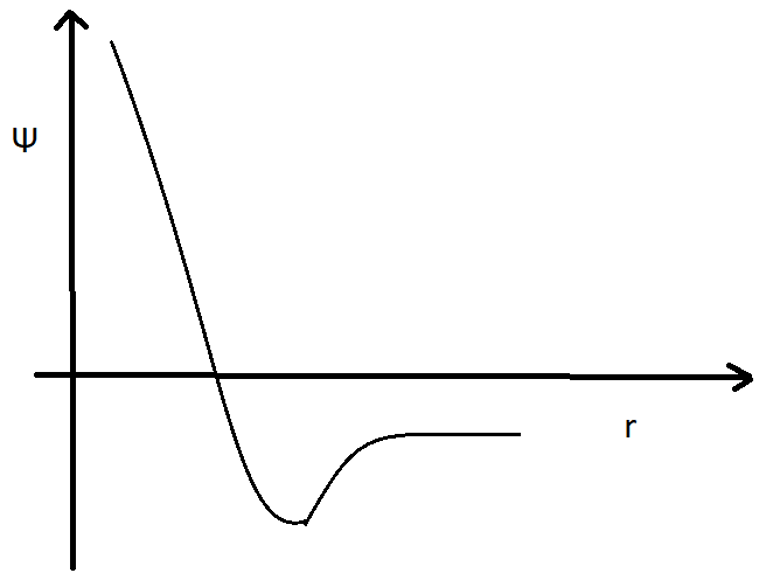
a) 1s
b) 2s
c) 2p
d) 3s
View Answer
Explanation: The given wave function is for the orbital 2s. It shows that after a certain distance from the nucleus, the graph touches the x-axis. Hence, it has a nodal plane.
14. Nodes are the plane where the probability of finding an electron is 1.
a) True
b) False
View Answer
Explanation: Nodal planes are described as the planes where the probability of finding an electron is not equal to zero. The total number of nodes in an orbital is n -1.
15. The probability of finding an electron is uniform in every direction is in which orbital?
a) s
b) p
c) d
d) p
View Answer
Explanation: For an s-orbital, the probability of finding an electron is uniform in every direction. For p orbitals, the probability of finding an electron is along one direction only.
Sanfoundry Global Education & Learning Series – Engineering Physics.
To practice all areas of Engineering Physics, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
