This set of Engineering Physics Multiple Choice Questions & Answers (MCQs) focuses on “PhotoElectric Effect”.
1. During Einstein’s Photoelectric Experiment, what changes are observed when the frequency of the incident radiation is increased?
a) The value of saturation current increases
b) No effect
c) The value of stopping potential increases
d) The value of stopping potential decreases
View Answer
Explanation: As the frequency of the incident radiation increases, the kinetic energies of the emitted electron increase as well and therefore requires more repulsive force to be applied to stop them. Thus, the stopping potential increases.
The value of saturation current increases, as the intensity of the incident radiation, increases.
The value of stopping potential decreases, as the frequency decreases.
2. What is the relation between the interaction parameter, ‘b’, and atomic radius, R, for the Photoelectric effect?
a) b > R
b) b ≈ R
c) b < R
d) no relation between b and R
View Answer
Explanation: If b > R, it means the interaction parameter is greater than the atomic radius. In this case, the electron is ejected by the photon and it is known as the Photoelectric effect.
If b ≈ R, the incident photons are scattered by the electron of the atom and the electron itself gets scattered. This phenomenon is known as the Compton effect.
If b < R, the photon is directly converted into an electron-positron pair, known as pair production.
3. What is the time lag between the incidence of photons and the ejection of photoelectrons?
a) Greater than 10-5 s
b) Between 10-5 s and 10-9 s
c) Less than 10-9 s
d) 1 second
View Answer
Explanation: The laws of photoelectric emission states that it is an instantaneous process because the photoelectric emission occurs due to the elastic collision between a photon and an electron. Practically, there is no time lag (< 10-9 s) between the incident photon and emission of an electron.
4. How does the intensity affect the photoelectric current?
a) As intensity increases, the photoelectric effect increases
b) As the intensity increases, the photoelectric effect decreases
c) As the intensity decreases, the photoelectric effect becomes twice
d) No effect
View Answer
Explanation: Since each incident photon ejects one photoelectron from a metal surface, therefore, the number of photoelectrons emitted depends on the number of photons falling on the metal surface, which in turn depends on the intensity on the incident light.
Hence, as the intensity increases, the number of photoelectrons ejected increases and hence photoelectric current increases.
5. The photoelectric emission could be explained by the ____________
a) Wave nature of light
b) Particle nature of light
c) Dual nature of light
d) Quantum nature
View Answer
Explanation: The wave theory of light could not explain the laws of photoelectric emission. It was only by particle nature of light that Einstein was able to explain the photoelectric emission.
It was considered that when a photon of incident radiation collides with an electron, it transfers its energy to the electron, thus causing photoelectric emission.
6. Identify the correct order of frequencies.
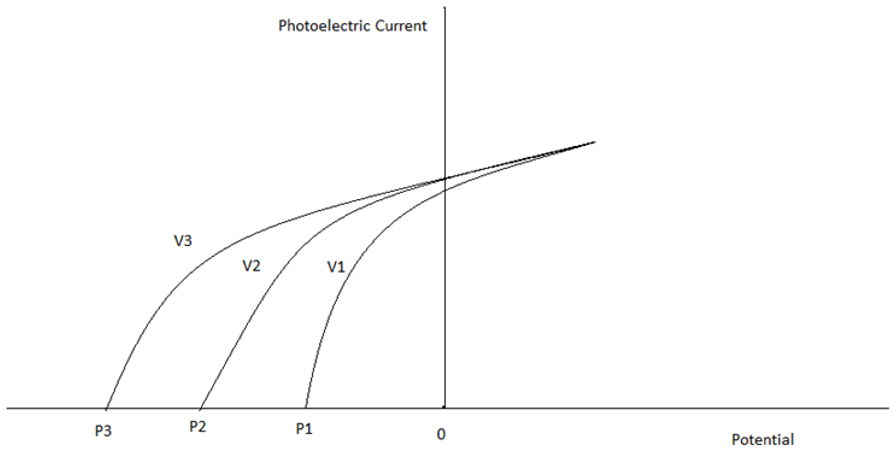
a) v1 > v2 > v3
b) v2 > v3 > v1
c) v3 > v2 > v1
d) v1 > v3 > v2
View Answer
Explanation: As the frequency of the incident radiation increases, the potential required to stop the ejected electron (Stopping Potential) increases. Hence, v3 > v2 > v1.
The stopping potential varies linearly with the frequency of the incident radiation.
7. The work function of lithium is 2.5 eV. The maximum wavelength of light that can cause the photoelectric effect in lithium is ______________
a) 3980 Å
b) 4980 Å
c) 5980 Å
d) 6980 Å
View Answer
Explanation: Work function, Φ0 = hv0 or \(\frac{hc}{λ_0}\)
Therefore, λ0 = \(\frac{hc}{\Phi_0} \)
Here, Φ0 = 2.5 eV = 2.5 X 1.6 X 10-19J
Maximum Wavelength, λ0 = \(\frac{hc}{\Phi_0} \) = 4.98 X 10-7 m
λ0 = 4980 Å.
8. Light of wavelength 3500 Å is incident on two metals A and B. Which metal will yield more photoelectrons if their work functions are 5 eV and 2 eV respectively?
a) A
b) B
c) A & B
d) C
View Answer
Explanation: Here, λ = 3500 Å = 3.5 X 10-7m
Energy of incident photons = hv = \(\frac{hc}{\lambda}\)
= 3.536 eV
Since the work function of metal A is higher, it will not yield any photoelectrons. Hence, only metal B will yield photoelectrons.
9. The Kinetic energy of a photoelectron emitted on shining a light of wavelength 6.2 X 10-6 m on a metal surface of work function 0.1 eV is _________________
a) 0.01 eV
b) 0.02 eV
c) 0.1 eV
d) 1 eV
View Answer
Explanation: Kinetic Energy of photoelectrons = hv – Φ0
= \(\frac{hc}{\lambda}\)– Φ0
Here, λ = 6.2 X 10-6m
Φ0 = 0.1 eV = 1.6 X 10-20J
Kmax = 3.2 X 10-20J – 1.6 X 10-20J
= 1.6 X 10-20J
= 0.1 eV.
10. What is the effect of intensity on the stopping potential?
a) As intensity increases, stopping potential increases linearly
b) As intensity increases, stopping potential decreases linearly
c) As intensity decreases, stopping potential increases exponentially
d) No effect
View Answer
Explanation: Changing the intensity of incident radiation does not affect the stopping potential. As the intensity increases, the number of photoelectrons ejected increases. However, the maximum velocity attained by them remains independent of the intensity of the radiation. It only depends on the frequency of the incident radiation.
11. Which of the following gases are filled inside the Photoelectric cells?
a) Carbon Dioxide
b) Nitrogen
c) Neon
d) Oxygen
View Answer
Explanation: Inert gases like neon or argon are filled inside the photoelectric cells to increase the photoelectric current due to ionization. When the potential difference between the two electrodes exceeds the ionization potential of the gas, the emitted photoelectrons ionize the gas which increases the magnitude of the current.
12. On which part of the photoelectric cell does the radiation strikes?
a) Cathode
b) Anode
c) Ammeter
d) Radiation does not strike on the photoelectric cell
View Answer
Explanation: The radiation strikes on the photosensitive plate which is used as a cathode. When radiation falls on the plate, photoelectrons are ejected which are accelerated towards charged plate A. This completes the circuit and the current flows.
13. For the photoelectric effect in sodium, the figure shows the plot of cut-off voltage versus frequency of incident radiation. The threshold frequency is __________
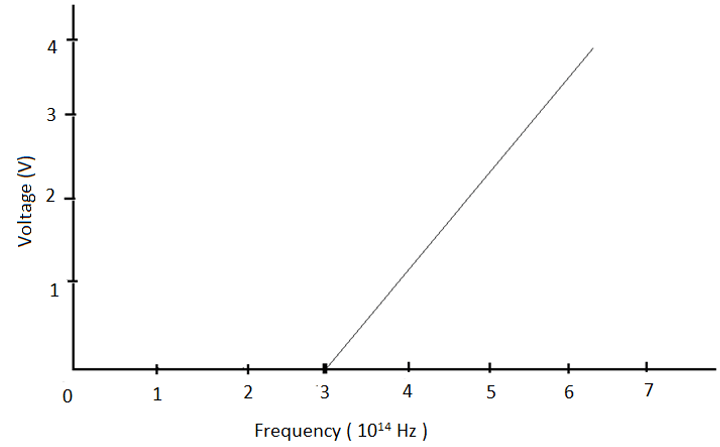
a) 6.5 X 1014 Hz
b) 4.5 X 1014 Hz
c) 3 X 1014 Hz
d) 5 X 1014 Hz
View Answer
Explanation: The threshold frequency corresponds to the frequency for which the cut-off voltage is zero. Hence, the threshold frequency for sodium, as observed in the graph, is 3 X 1014 Hz.
Sanfoundry Global Education & Learning Series – Engineering Physics.
To practice all areas of Engineering Physics, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
