This set of Engineering Physics Multiple Choice Questions & Answers (MCQs) focuses on “Nuclear fission & Nuclear Reactor”.
1. Nuclear fission is the phenomenon of ___________
a) Heavy nucleus splitting
b) Heavy nucleus combining
c) Light nucleus splitting
d) Light nucleus combining
View Answer
Explanation: Nuclear fission is the phenomenon of splitting of a heavy nucleus into lighter nuclei. The nucleus generally has a mass number greater than 230.
2. The Q-value of fission reaction is of the order ___________
a) 10 MeV
b) 100 MeV
c) 200 MeV
d) 500 MeV
View Answer
Explanation: The Q-value of a reaction is the energy released in a fission reaction. It is of the order 200MeV per fissioning nucleus.
3. An atom bomb is a ____________
a) Controlled fission reaction
b) Controlled fusion reaction
c) Uncontrolled fission reaction
d) Uncontrolled fusion reaction
View Answer
Explanation: A chain reaction is a series of fission reactions. In an atom bomb, an uncontrolled chain reaction proceeds while in a nuclear reactor a controlled chain reaction is produced.
4. The average energy of a neutron produced in a fission of Uranium 235 isotope is ___________
a) 1 MeV
b) 2 MeV
c) 10 MeV
d) 100 MeV
View Answer
Explanation: The average energy of a neutron produced in fission of a uranium 235 isotope is 2 MeV. These neutrons unless slowed down will escape from the reactor without interacting with uranium nuclei.
5. The moderator commonly used is ___________
a) Diamond
b) Graphite
c) Lead
d) Copper
View Answer
Explanation: The most commonly used moderators are water, heavy water and graphite. Moderators are used to control the chain-reaction and slow down the neutrons.
6. When the multiplication factor is greater than 1, the power increases linearly.
a) True
b) False
View Answer
Explanation: When the multiplication factor becomes greater than 1 in a Nuclear Reactor, the reaction rate and the reactor power increases exponentially. Unless bought down, the reactor will become supercritical and explode.
7. Reflectors are used in a nuclear reactor to control the speed of neutrons.
a) True
b) False
View Answer
Explanation: Reflectors are present in a nuclear reactor to reduce leakage. Moderators are used to slow down the incoming neutrons from the core.
8. The safety-rods present to shut down the reactor are made up of ____________
a) Copper
b) Calcium
c) Carbon
d) Cadmium
View Answer
Explanation: The safety-rods are present in a nuclear reactor to shut it down immediately in the case of an emergency. They are made up of neutron-absorbing material such as cadmium.
9. The neutrons slowed down by moderators are called as ____________
a) Slowed neutrons
b) Thermal neutrons
c) Fission neutrons
d) Moderated neutrons
View Answer
Explanation: The neutrons which are slowed down by the moderators are referred to as thermal neutrons. The neutron slow down after colliding with the moderators, which are made up of water, heavy water etc.
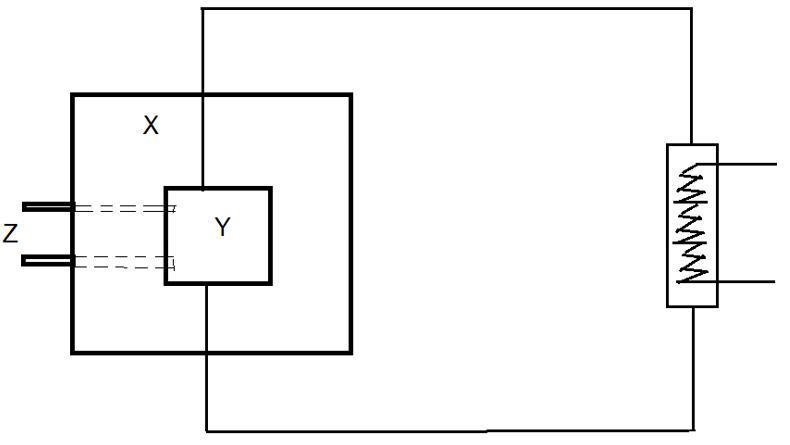
10. Following is the diagram of a nuclear reactor. Identify X.
a) Control Rods
b) Core
c) Reflectors
d) Coolant
View Answer
Explanation: In the given figure, X is the reflectors, which helps in reducing the leakage, z are the control rods, present for safety, and Y is the core, where the nuclear fission reaction takes place.
11. How many fission takes place per second in a 300 MW reactor, assuming each fission releases 200 MeV of energy?
a) 2.38 X 1018
b) 4.38 X 1018
c) 8.38 X 1018
d) 9.38 X 1018
View Answer
Explanation: Let n bet the number of fissions.
Energy released per second = 200 X 106 X 1.6 X 10-19 X n J
= n X 3.2 X 10-11 J
Power = Energy/Time
300 X 106 = n X 3.2 X 10-11
n = 9.38 X 1018.
12. The fission of a single nucleus of 235U releases 200 MeV of energy. What is the number of fissions that must occur to produce a power of 1 MW?
a) 3.23 X 1016
b) 3.12 X 1016
c) 4.38 X 1016
d) 5.38 X 1016
View Answer
Explanation: Energy released per second = n X 200 MeV
= n X 200 X 1.6 X 10-19 J
Energy produced per second = Power X time
= 1 MW X 1s
n X 200 X 1.6 X 10-19 J = 106 J
n = 3.12 X 1016.
13. Calculate the energy released by the fission of 5 gm of 235U in kWh if energy released per fission is 200 MeV.
a) 1.13 X 105 kWh
b) 2.65 X 105 kWh
c) 3.78 X 105 kWh
d) 4.12 X 105 kWh
View Answer
Explanation: Now, Number of atoms in 5 g of 235U = 6.023 X 1023 X 5/235
Energy released by the fission = 6.023 X 1023 X 5 X 200 X 1.6 X 10-19 / 235
= 4.1 X 1011J
1.13 X 105 kWh.
14. If the number of fissions are 2.375 X 1024, what is the mass of uranium 235 isotope used?
a) 789.23 g
b) 879.32 g
c) 926.65 g
d) 980.74 g
View Answer
Explanation: Mass of uranium used, m = \(\frac{Mass \,Number\, X n}{Avogadro’s\, number}\)
= \(\frac{235 X 2.375 X 10}{6.023}\)
= 926.65 g.
15. At high temperatures, which material is the best choice to be used as coolant in a nuclear reactor?
a) Water
b) Heavy water
c) Molten Potassium
d) Molten Carbon
View Answer
Explanation: Water and heavy water are good coolants, but due to their low boiling points, they need pressurization. At high temperature, liquid metals like molten sodium or potassium are excellent coolants.
Sanfoundry Global Education & Learning Series – Engineering Physics.
To practice all areas of Engineering Physics, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
