This set of Engineering Physics Multiple Choice Questions & Answers (MCQs) focuses on “Carbon Nanostructures”.
1. The configuration of Buckminsterfullerene is ____________
a) 12 Hexagons and 22 Pentagons
b) 18 Hexagons and 15 Pentagon
c) 20 Hexagon and 12 Pentagon
d) 15 Hexagon and 15 Pentagon
View Answer
Explanation: Buckminsterfullerene, commonly known as fullerene, consists of 20 hexagons and 12 pentagons, which are arrayed such that no two pentagons share a common side.
2. C60 is soluble in ____________
a) Water
b) Ammonia
c) HCl
d) Benzene
View Answer
Explanation: C60 molecules are held together by weak Van der Waal’s forces. They are insoluble in ionic solvents like water, ammonia and HCl, as it is non-ionic. As it is an organic molecule, it is soluble in Benzene.
3. CNTs stands for ____________
a) Carbon Nanotubes
b) Carbon Nanotechnology
c) Carbon Nanoscience and technology
d) Carbon Nine Technology
View Answer
Explanation: Carbon nanotubes, CNTs, are nanostructures with large application potential. Its structure consists of a single sheet of graphite rolled into a tube.
4. CNTs are capped on both ends with which carbon nanostructure?
a) Graphite
b) Diamond
c) C60
d) Benzene
View Answer
Explanation: Carbon Nanotubes are basically a single sheet of graphite, which is rolled into a tube. The ends of the CNTs are covered by the C60 hemispheres. Such is the construction of a CNT.
5. For the synthesis of CNTs, the quartz tube is heated up to ____________
a) 1000℃
b) 1200℃
c) 1400℃
d) 1600℃
View Answer
Explanation: For the synthesis of CNTs, a quartz tube containing argon gas and a graphite target are heated to 1200o C. This process takes place by the use of lasers.
6. The metallic tubes have which kind of structure?
a) Armchair
b) Chiral
c) Boat
d) Achiral
View Answer
Explanation: The CNTs are often a mixture of semiconducting and metallic tubes. While the metallic tubes have armchair structure, the semiconducting tubes have a chiral structure.
7. The carbon tubes have high conductivity.
a) True
b) False
View Answer
Explanation: The reason for the high conductivity of the carbon nanotubes is that they have very few defects to scatter electrons and therefore show very low resistance.
8. Carbon nanotubes display magnetoresistance at low temperature.
a) True
b) False
View Answer
Explanation: Magnetoresistance is the phenomenon in which the resistance of a material changes on the application of a magnetic field. Carbon nanotubes display this property at low temperature. In fact, it shows negative magnetoresistance as the resistance decreases with an increasing magnetic field.
9. Identify the process.
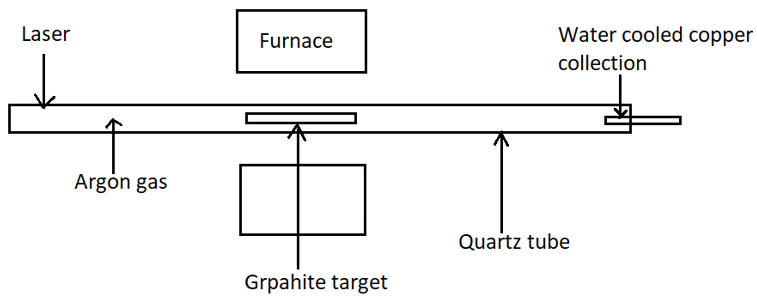
a) Synthesis of C60
b) Synthesis of CNTs
c) Chemical vapour deposition
d) Carbon discharge
View Answer
Explanation: The given figure shows the schematic diagram for synthesis of CNT by laser evaporation. The quartz tube is heated to 1200°. A water called copper collection is present at the end to cool it down.
10. The main purpose of CNTs in fuel cells is ___________
a) Production of energy
b) Active medium
c) Catalyst
d) Storage
View Answer
Explanation: Carbon nanotubes are useful in fuel cells as well as in batteries, primarily for storage purposes of hydrogen. The tubes need to gold 6.5% hydrogen by weight.
11. Carbon nanotubes are poor transmitters of electromagnetic radiations due to their ____________
a) High conductivity
b) Large surface area
c) High porosity
d) Chemical Stability
View Answer
Explanation: Carbon nanotubes have very high electrical conductivity. Due to this, they are poor transmitters of electromagnetic radiations. A plastic composite of CNTs could provide lightweight shielding material for electromagnetic radiation.
12. The figure shows a FET using CNT. Identify CNT.
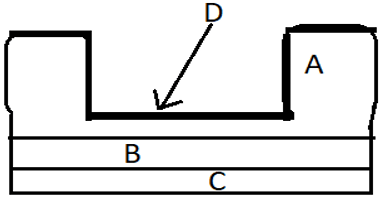
a) A
b) B
c) C
d) D
View Answer
Explanation: In the diagram, A is the source and the drain (gold), B is the insulating layer of silicon dioxide, C is the gat made of silicon substrate and D is the CNT. CNTs are useful as the new generation interconnect structures as well as FETs.
Sanfoundry Global Education & Learning Series – Engineering Physics.
To practice all areas of Engineering Physics, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
