This set of Drug Biotechnology Multiple Choice Questions & Answers (MCQs) focuses on “Pharmacokinetic Models”.
1. Which of the following is not a mechanism for pharmacokinetic analysis?
a) Compartment analysis
b) Non compartment analysis
c) Physiologic modeling
d) Human model
View Answer
Explanation: There are three different approaches to the pharmacokinetic analysis of experimental data. These are compartment modeling, Noncompartment modeling, physiological modeling. There no such thing called a human model.
2. In which of the following models the body is considered to be composed of several compartments?
a) Compartment model
b) Noncompartment model
c) Physiologic model
d) Human model
View Answer
Explanation: In the compartmental model, the body is considered to be composed of several compartments. Tissues which are approximately similar in drug distribution characteristics form a single compartment. These compartments are imaginary and not physiologic or anatomic region.
3. In which of the model peripheral compartments are connected to a central compartment?
a) Compartment model
b) Caternary model
c) Physiologic model
d) Mammillary model
View Answer
Explanation: Mammillary model is the most common compartment model used in pharmacokinetics. This method has one or more peripheral compartments connected to the central compartment. The central compartment comprises of plasma and highly perfused tissue such as lungs, livers, kidneys, etc.
4. Which organs will make up the peripheral compartment?
a) Lungs
b) Liver
c) Kidneys
d) Pancreas
View Answer
Explanation: The central compartment consists of highly perfused tissues such as that of lungs, kidneys, liver, etc. The peripheral compartment or tissue compartment consists of organs which are of low vascularity and poor perfusion.
5. What type of drug administration will have the shown compartment model?

a) Intravenous administration
b) Oral administration
c) Rectal administration
d) Sublingual administration
View Answer
Explanation: There is no arrow entering the 1st compartment itself, this means whatever the administration is it directly goes into the blood. In intravenous administration, the blood is considered to be the 1st compartment. Since one single compartment is shown it will be intravenous administration. K10 is the first order elimination rate constant.
6. What type of drug administration will have the shown compartment model?

a) Intravenous administration
b) Oral administration
c) Rectal administration
d) Sublingual administration
View Answer
Explanation: There is no arrow entering the 1st compartment itself, this means whatever the administration is it directly goes into the blood. In intravenous administration, the blood is considered to be the 1st compartment, this blood will be moving to organs like lungs, kidneys, liver, etc. Since one single compartment is shown it will be intravenous administration. Ko1 is the rate constant, i.e. the first order absorption rate constant and K10 is the first order elimination rate constant. The 2nd compartment is for the organs with poor perfusion.
7. What type of drug administration will have the shown compartment model?

a) Intravenous administration
b) Oral administration
c) Rectal administration
d) Extravascular administration
View Answer
Explanation: There is an arrow entering the 1st compartment, thus this one compartment model will be for extravascular administration such as oral, rectal, vaginal, etc. The 1st compartment is shown for the blood which will be passing through the liver, lungs, kidneys, etc. Ko1 is the rate constant, i.e. the first order absorption rate constant and K10 is the first order elimination rate constant.
8. What type of drug administration will have the shown compartment model?

a) Intravenous administration
b) Oral administration
c) Rectal administration
d) Sublingual administration
View Answer
Explanation: There is an arrow entering the 1st compartment. Thus this two-compartment model is for extravascular administration. The 2nd compartment is for the organs with poor perfusion. Ko1 is the rate constant, i.e. the first order absorption rate constant and K10 is the first order elimination rate constant. K12 and K21 is the rate constant for the absorption into the organs of the 2nd compartment and elimination from the 2nd compartment organs.
9. In which model compartments are joined in series?
a) Compartment model
b) Caternary model
c) Physiologic model
d) Mammillary model
View Answer
Explanation: In Caternary model, the compartments are joined to one another in a series like compartments. This is not observable anatomically since various organs are directly linked to the blood compartment. Mammillary model is the most common compartment model used in pharmacokinetics. This method has one or more peripheral compartments connected to the central compartment.
10. Which of the following is not a characteristic of the caternary compartment model?
a) It gives a visual representation of various rate processes in drug disposition
b) It shows how many rate constants are necessary
c) Compartments and parameters bear a relationship with physiologic functions
d) Useful in predicting drug
View Answer
Explanation: In the caternary model, compartments and parameters are not in a relationship with the physiological functions of the species. This is a disadvantage of the caternary model. Caternary model gives a visual representation of various rate processes, it shows how many rate constants are necessary, etc.
11. In noncompartmental analysis, Mean residence time is equal to _____________
a) The area under the first moment curve/area under the zero moment curve
b) The area under the zero moment’s curve/area under the first moment curve
c) 1 / Area under the first-moment curve
d) 1/ Area under the zero moment curve
View Answer
Explanation: For noncompartmental analysis, the mean residence time is equal to the area under the first moment curve (AUMC) / area under the zero moment curve (AUC). AUMC is obtained from a plot of plasma drug concentration and time versus time t.
12. Which pharmacokinetic model is drawn on the basis of anatomic and physiologic data?
a) Compartment model
b) Caternary model
c) Physiologic model
d) Mammillary model
View Answer
Explanation: The physiologic model is drawn on the basis of anatomic and physiologic data. Thus, it represents a more realistic picture of drug disposition in various organs and tissues. The number of compartments to be added depends on the drug that how many organs the drug will get distributed to.
13. Which kind of pharmacokinetic model is shown in the picture?
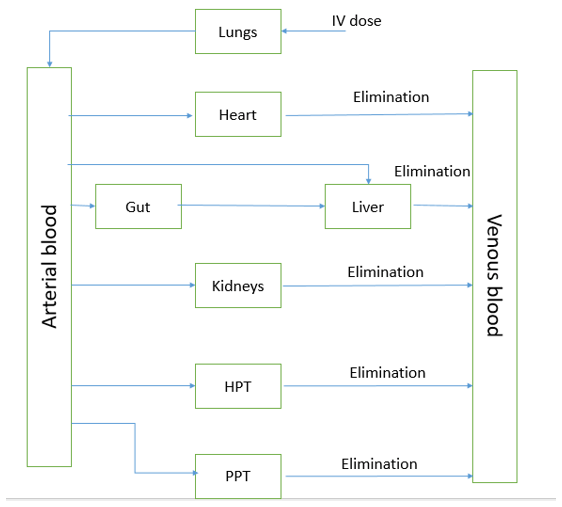
a) Physiologic model
b) Compartment model
c) Noncompartment model
d) Mammillary model
View Answer
Explanation: In this model we can see compartments depending on each organ. The physiologic model is made on the basis of known anatomic and physiologic data, it presents a more realistic idea of drug disposition in various organs. Organs which have no drug disposition are excluded and organs, where drug disposition occurs, are taken into account.
14. Which of the following will be a disadvantage for the physiologic model?
a) Prediction of drug concentration in various body regions
b) Correlation of data in several animal species
c) Obtaining experimental data for each of the organs
d) The model gives an exact description of the drug concentration-time profile for any organ
View Answer
Explanation: The advantages of physiologic model are, concentration of the drug in various body parts can be predicted on the basis of organ or tissue volume, the model gives an exact description of the drug concentration-time profile for any organ, correlation of data in several animal species is possible and the disadvantage is that obtaining experimental data for each an every organ is difficult.
15. Which model is also known as membrane permeation rate limited?
a) Physiologic model
b) Compartment model
c) Noncompartment model
d) Mammillary model
View Answer
Explanation: Physiologic models are made with the assumption that the drug movement within a body region is much more rapid than its rate of delivery to that region, this assumption will be applicable to highly membrane permeable drugs i.e. drugs with low molecular weight, nonionized and lipophilic drugs. For highly polar, ionized and charged drugs, the model is referred to as membrane permeation rate-limited.
Sanfoundry Global Education & Learning Series – Drug and Pharmaceutical Biotechnology.
To practice all areas of Drug and Pharmaceutical Biotechnology, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Check Drug and Pharmaceutical Biotechnology Books
- Check Biotechnology Books
- Practice Biotechnology MCQs
- Apply for Biotechnology Internship
