This set of Class 11 Chemistry Chapter 3 Multiple Choice Questions & Answers (MCQs) focuses on “Modern Periodic Law and the Present Form of the Periodic Table”.
1. ____________ observed the X-rays’ characteristics.
a) Henry Moseley
b) Mendeleev
c) Pauli
d) Newland
View Answer
Explanation: Henry Moseley was an English physicist whose is know for modification of Mendeleev’s periodic table as he found out that atomic number is more prominent than atomic weight by his observation in regularities in characteristics in X-rays.
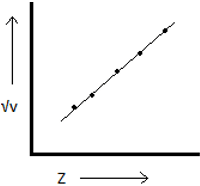
2. The graph of \(\sqrt{v}\) Vs Z gives a straight line.
a) True
b) False
View Answer
Explanation: In the above statement v represents the frequency of X-rays emitted and Z depicts atomic number, but not the mass number. Henry Moseley found out that atomic number is more prominent than atomic weight by this graph.
3. The physical and chemical properties of an element are the periodic function of its _________
a) Atomic mass
b) Element behaviour
c) No of electrons
d) Atomic number
View Answer
Explanation: The above statement that the periodic function of the atomic number states the physical and chemical properties of that element is the modern periodic law is changed according to Henry Moseley by these experiments.
4. What does Z represent in the above graph?
a) Atomic mass
b) Atomic number
c) Frequency
d) Wavelength
View Answer
Explanation: The above graph states Henry Moseley’s law, where v represents the frequency of X-rays emitted and Z depicts atomic number. It states that the atomic number is more prominent than atomic weight in case of periodic classification.
5. Henry Moseley’s law is given by the equation \(\sqrt{v}\) = a(Z – b). What do a and b represent here?
a) The proportionality constant, screening constant
b) Screening constant, proportionality constant
c) Absorption constant, proportionality constant
d) screening constant, Boltzmann constant
View Answer
Explanation: In the equation \(\sqrt{v}\) = a(Z – b), a represents proportionality constant and b represents screening constant.a and b are independent by not depending on the target’s nature. The above equation is the result of Henry Moseley’s law of the modern periodic table.
6. The horizontal rows in Mendeleev’s periodic table were called as __________
a) periods
b) groups
c) series
d) rows
View Answer
Explanation: A Russian chemist named Dmitri Mendeleev classified elements based on chemical and physical properties in ascending order of their atomic weights. The number of groups was 8 (vertical columns) and there were 12 series (horizontal rows).
7. In the modern long-form of periodic table, the horizontal rows and the vertical columns are called as __________ and ___________ respectively.
a) groups, periods
b) periods, groups
c) rows, columns
d) columns, rows
View Answer
Explanation: In the most convenient and widely used periodic table of the long-form that is the modern version, the horizontal rows and the vertical columns are called as periods (series as per Mendeleev) and groups respectively.
8. The period’s number corresponds to the highest _________
a) Azimuthal quantum number
b) Spin quantum number
c) Magnetic quantum number
d) Principal quantum number
View Answer
Explanation: As seen in the most convenient and widely used periodic table of the long-form that is the modern version, the horizontal rows that depict period number represent the highest principal quantum number of the atoms in the period.
9. Which was the incomplete period in the long-form of the modern periodic table?
a) 7th period
b) 4th period
c) 6th period
d) 2nd period
View Answer
Explanation: The 7th period follows the rule that the elements fill their 7s subshell first and then 5f, 6d and 7p (Plutonium is the exception). As the elements weren’t discovered completely, it was called the incomplete period.
10. Which of the following period is the shortest one?
a) 1
b) 3
c) 2
d) 4
View Answer
Explanation: The first period is known as the shortest period among the seven periods of the periodic table this is because it contains only two elements; one of the two elements is hydrogen which belongs to alkali metal group and the other is the Helium which is a noble gas.
Sanfoundry Global Education & Learning Series – Chemistry – Class 11.
To practice all chapters and topics of class 11 Chemistry, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Practice Class 11 - Physics MCQs
- Practice Class 11 - Biology MCQs
- Check Class 11 - Chemistry Books
- Practice Class 12 - Chemistry MCQs
- Practice Class 11 - Mathematics MCQs
