This set of Class 11 Chemistry Chapter 2 Multiple Choice Questions & Answers (MCQs) focuses on “Discovery of Sub-atomic Particles”.
1. Which of the following may be an incorrect statement regarding cathode ray discharge tube?
a) Presence of negative charge in cathode rays
b) The magnetic field deflects these rays
c) It needs high voltage
d) Protons are present in cathode rays
View Answer
Explanation: Electric discharge in partially evacuated tubes in cathode ray discharge tube. Cathodes rays are made up of electrons. There is a presence of negative charge in cathode rays, magnetic field deflects these rays. Cathode ray discharge tube needs high voltage.
2. Pick out electron’s charge to mass ratio’s value from the options.
a) 1.758820 × 1011 C kg-1
b) 1.758820 × 1011 C kg
c) 1.758823 × 1011 C kg
d) 1.708820 × 1011 C kg
View Answer
Explanation: A British physicist J. J. Thomson carried out experiments and observed the deflections made by electrons in an electric or magnetic field. He finally calculated electron’s charge to mass ratio as e/me = 1.758820 × 1011 C kg-1. The units of charge and mass are in coulomb and kg respectively.
3. Which of the following condition is suitable for cathode ray discharge tube?
a) Low pressure, high voltage
b) Low pressure, low voltage
c) High pressure, low voltage
d) High pressure, high voltage
View Answer
Explanation: The suitable conditions of cathode ray discharge tube are low pressure and high pressures. Pressure can be adjusted by evacuated tubes. High voltage is applied across electrodes and current starts flowing through the tube.
4. Who did the oil drop experiment?
a) R. A. Millikan
b) J. J. Thomson
c) Rutherford
d) Galileo
View Answer
Explanation: R. A. Millikan conducted oil drop experiment to measure the mass of oil droplets. After observation of how the charge is transferred in the experiment he concluded that charge only appears in integral multiples of e i.e. q = ne; n = ±1, ±2, ±3, etc.
5. Thomson discovered that every substance in this universe is made up of ________ from his experiments.
a) neutrons
b) protons
c) electrons
d) mass
View Answer
Explanation: Through cathode rays discharge tube experiments, he concluded that every substance in this universe is made up of electrons from his experiments. He observed how cathode rays move and the charge to mass ratio of it.
6. When cathode rays strike zinc sulfide coating, what did it create?
a) bright spot
b) blue light
c) uv rays
d) white light
View Answer
Explanation: Zinc sulfide is a phosphorescent material, which has been coated on the anode. when cathode rays strike on the anode, electrons hit on the Zinc sulfide screen, hence creating a bright spot.
7. ___________ is the lightest and smallest particles that’s obtained from hydrogen(that is a positive ion).
a) Electron
b) Proton
c) Neutron
d) Particle
View Answer
Explanation: In 1911, Rutherford discovered protons by performing experiments and also found out that positive charge is concentrated at centre and that it has most of the atomic mass. The name proton was first time given in the year 1920.
8. What’s the mass of neutron in terms of electrons mass?
a) 1838 times of electron’s mass
b) 1/1838 times of electron’s mass
c) 1832 times of electron’s mass
d) 1/1832 times of electron’s mass
View Answer
Explanation: The mass of an electron is 0.00054 u. The mass of a proton is 1.00727 u. The mass of a neutron is 1.00867 u. Then mass of neutron by mass of an electron is 1.00867/0.00054 = 1838. Therefore the mass of neutron in terms of the electron is 1838 times.
9. Neutrally charged particles are protons.
a) True
b) False
View Answer
Explanation: Neutrally charged particles are known as neutrons, whereas positively charged particles are protons. When Beryllium is bombarded by alpha particles, neutrons were discovered by Chadwick. Their mass is a bit greater than that of protons.
10. The below model of organization of electrons in atom is given by ___________
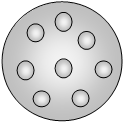
a) R. A. Millikan
b) J. J. Thomson
c) Rutherford
d) Galileo
View Answer
Explanation: Thomson proposed a model of the atom, in which electrons are embedded to make it as the stable electrostatic arrangement and such that positive charge is equally distributed around a sphere. Mass is assumed to be equally distributed. So. it has different names like plum pudding, watermelon and raisin pudding model.
Sanfoundry Global Education & Learning Series – Chemistry – Class 11.
To practice all chapters and topics of class 11 Chemistry, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Check Class 11 - Chemistry Books
- Practice Class 11 - Physics MCQs
- Practice Class 11 - Mathematics MCQs
- Practice Class 11 - Biology MCQs
- Practice Class 12 - Chemistry MCQs
