This set of Class 11 Chemistry Chapter 5 Multiple Choice Questions & Answers (MCQs) focuses on “States of Matter – Gas Laws”.
1. At a constant temperature, the pressure of a gas is given as one atmospheric pressure and 5 liters. When the atmospheric pressure is increased to 2 atm, then what is the volume of the gas?
a) 1 liter
b) 5 liters
c) 10 liters
d) 2.5 liters
View Answer
Explanation: As we know that, Boyle’s law states at a constant temperature, the pressure of a gas is inversely proportional to its volume so P1V1 equals to P2V2 by substituting P1 as one atmospheric pressure V1 as 5 liters P1 as to atmospheric pressure we get V2 as 5/2 that is 2.5 liters.
2. What is the shape of the graph that is drawn between pressure and volume?
a) A straight line
b) Circular
c) Parabola
d) Hyperbola
View Answer
Explanation: Boyle’s law states that at constant temperature pressure is inversely proportional to the volume of gas so here the graph between the pressure as y-axis volume as x-axis is in the shape of a hyperbola.
3. What is the name of the graph that is drawn, when the temperature is kept constant?
a) Isotherm
b) Isochoric and isobar
c) Isochoric
d) Isobar
View Answer
Explanation: The graphs with constant temperature plot are isotherms. For example, the graph that is used to detect the Boyle’s law, that is between pressure and volume is an isotherm as the temperature is constant in this graph.
4. There is a ball that will burst if the pressure exceeds 0.12 bars. The pressure of the gas is 1 bar and the volume is 2.5 liters. What can be the maximum volume that the ball can be expanded?
a) 0.12 liters
b) 2.5 liters
c) 0.3 liters
d) 1 liter
View Answer
Explanation: According to Boyle’s law at a constant temperature, the pressure is inversely proportional to the temperature so here P1V1 is equaled to P1V2 by equating P1V1 is equaled to 1 x 2.5 = 2.5, so the maximum volume of the ball that can be expanded is 2.5/0.12 =0.3 liters.
5. How much does the volume of the gas increase if we increase the temperature by 1 Degree?
a) 273 liters
b) 1 by 273rd of the original volume of the gas
c) 1 liter
d) Hundred liters
View Answer
Explanation: According to Charles law, the volume of the fixed gas at constant pressure is directly proportional to the Absolute Temperature of the gas. So we thereby represent this as Vt = V0(1 + t/273) where Vt is the volume of the gas at temperature t and V0 is the volume of the gas at 0 degrees Celsius that is absolute temperature.
6. There is a balloon filled with a gas at 26-degree centigrade and has a volume of about 2 liters when the balloon is taken to a place which is at 39-degree centigrade, what would be the volume of the gas that is inside the balloon?
a) 2 liters
b) 3 liters
c) 1.5 liters
d) 0.67 liters
View Answer
Explanation: As we know that temperature is directly proportional to the volume at constant pressure, 26/39 = 2/ X; so here by equating X equals to 3 liters. Hence required a volume of the balloon at 39 degrees is 3 liters.
7. By observing the below-given figure which of the options do you think is the correct one?
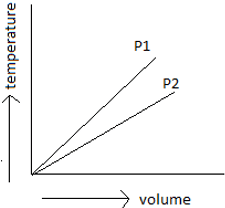
a) P1 is greater than P2
b) P2 is greater than P1
c) P1 is equal to P2
d) P1 may be equal to P2
View Answer
Explanation: By using Boyle’s law, draw a parallel line to volume axis, so as to maintain a constant temperature. Draw perpendicular lines to the point of intersection of pressure lines to constant temperature and volume axis. Now see to the lower the volume, higher the pressure. So P1 is greater than P2.
8. An ideal gas of 10 moles occupies _________ volume.
a) 22.4 liters
b) 2.24 liters
c) 224 liters
d) 2240
View Answer
Explanation: As we know that the number of moles is proportional to the volume as per Avogadro’s law and we also know that an ideal gas at STP occupies 22.4 liters of volume. So here 10 moles of gas occupies 224 liters of volume.
9. When a graph is drawn between the pressure and temperature of the gas it is known as _________
a) isochoric
b) isobar
c) isotherm
d) isotopic
View Answer
Explanation: When a graph is plotted between pressure on y-axis and temperature on x-axis straight line is formed at a constant volume and this graph is known as isochoric. As we know that gay lussac’s law proposes that at the constant volume the pressure and temperature are directly proportional.
10. At 22 degree Celsius a gas consists of pressure 1.1 bars then what is the temperature when the gas consists a pressure of 2.2 bars?
a) 11 degree Celsius
b) 44 degree Celsius
c) 33 degree Celsius
d) 22 degree Celsius
View Answer
Explanation: According to Gay-Lussac’s law, at the constant volume, the pressure is directly proportional to the temperature of gas so P1/P2 = T1/T2 that is 1.1 Bar/2.2 bar = 22 degrees Celsius/44 degree Celsius. So the temperature required is 44 degrees Celsius.
Sanfoundry Global Education & Learning Series – Chemistry – Class 11.
To practice all chapters and topics of class 11 Chemistry, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Practice Class 11 - Physics MCQs
- Practice Class 11 - Mathematics MCQs
- Check Class 11 - Chemistry Books
- Practice Class 12 - Chemistry MCQs
- Practice Class 11 - Biology MCQs
