This set of Chemical Process Calculation Multiple Choice Questions & Answers (MCQs) focuses on “Pressure and Hydrostatic Head-I”.
1. The normal force per unit area that a fluid exerts on a surface is called
a) Normal Force
b) Pressure
c) Gauge pressure
d) None of the mentioned
View Answer
Explanation: The normal force per unit area that a fluid exerts on a surface is called pressure.
2. Find out the correct statement
a) Pabsolute = Pgauge – Patmospheric
b) Pvacuum = Patmospheric + Pabsolute
c) Both and b are incorrect
d) Both and b are correct
View Answer
Explanation: Pabsolute = Pgauge + Patmospheric and Pvacuum = Patmospheric – Pabsolute.
3. Pressure can`t be expressed as
a) N/m2
b) Pa/m2
c) atm
d) mm of Hg
View Answer
Explanation: Pa is the unit of pressure, not Pa/m2.
4. 1 atm pressure is equal to
a) 101.3 kPa
b) 760 mm of Hg
c) 14.69 psi
d) All of the mentioned
View Answer
Explanation: 1 atm = 101.3 kPa = 760 mm of Hg = 14.69 psi.
5. A static column of height h is filled with a liquid of density D. What is the static pressure at the bottom if the column is placed in open.
a) P* + Dgh
b) Dgh
c) P*
d) None of the mentioned
View Answer
Explanation: Static pressure at the bottom = P* + Dgh.
6. For the given manometer
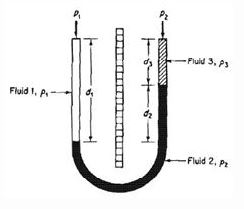
a) P1 + ρ1d2g = P2 + ρ2d2g
b) P1 + ρ1d1g = P2 + ρ2d2g
c) P1 + ρ1d1g = P2 + ρ2d2g + ρ3d3g
d) None of the mentioned
View Answer
Explanation: P1 + ρ1d2g = P2 + ρ2d2g + ρ3d3g. For same liquid at same level, pressure will be always same.
7. A bucket is placed in vacuum that contains a 14 kg/m3 density fluid. The bucket is filled with a height of 5 m. What is the static pressure at the bottom of the bucket?
a) 484 Pa
b) 686 Pa
c) 888 Pa
d) 1000 Pa
View Answer
Explanation: Pressure = Dgh = 14*9.81*5.
8. Two statement are given as below
I: 35 psia = 71.24 in Hg
II: Barometer is used for the measurement of vacuum pressure
a) I is correct and II is incorrect
b) II is correct and I is incorrect
c) Both are correct
d) Both are incorrect
View Answer
Explanation: Barometer is used for the measurement of atmospheric pressure.
9. In barometer, the given space labelled as A, above the mercury is

a) Vacuum
b) Filled with Air
c) Filled with water
d) Filled with Gas
View Answer
Explanation: To correctly measure the atmospheric pressure, the space above the mercury level in a barometer is essentially vacuum. Otherwise, the fluid in the space will exert a pressure on the mercury surface.
10. In the previous question
Air is trapped in the space (labelled A) above the mercury level in the tube of a barometer, which measures 750 mm Hg. If the atmospheric pressure is 760 mm Hg, then the pressure of the trapped air is
a) 15 mm Hg
b) 760 mm Hg
c) Between 745 and 760 mm Hg
d) 745 mm Hg
View Answer
Explanation: Pressure of trapped air + Pressure of mercury column = Atmospheric Pressure.
Sanfoundry Global Education & Learning Series – Chemical Process Calculation.
To practice all areas of Chemical Process Calculation, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Check Chemical Process Calculations Books
- Check Chemical Engineering Books
- Apply for Chemical Engineering Internship
- Practice Chemical Engineering MCQs
