This set of Chemical Process Calculation Multiple Choice Questions & Answers (MCQs) focuses on “Material Balance without Reaction-I”.
1-2. A membrane is used for separation of gases from waste. If the feed stream contains 20% CO2 and 80% SO2 and the product contains 25% CO2 and 75% SO2. Assume that the waste stream amounts to 80% of the input stream.
1. What is the composition of CO2 in waste stream?
a) 0.1875
b) 1.1685
c) 0.1485
d) 0.1285
View Answer
Explanation: Mole balances for both the gases.
2. What is the composition of SO2 in waste stream?
a) 0.8175
b) 0.8165
c) 0.8145
d) 0.8125
View Answer
Explanation: Mole balances for both the gases.
3. Below given statements for distillation is
In distillation, a liquid mixture is boiled to produce a vapour of different composition that moves away from the liquid.
a) True
b) False
c) Partially correct
d) None of the mentioned
View Answer
Explanation: In distillation, a liquid mixture is boiled to produce a vapour of different composition that moves away from the liquid.
4-5. In a distillation column for a feed of1000 kg the output is 80% of the feed and remaining 20% is waste. The feed contains 20% of Acetone and rest Water. The product contains 20% of Acetone and 40% of Water.
4. How much Acetone is there in waste?
a) 20 kg
b) 40 kg
c) 60 kg
d) 80 kg
View Answer
Explanation: Mass balance for each component.
5. Moles of water in waste?
a) 4.45
b) 8.89
c) 16.67
d) 20.48
View Answer
Explanation: Mass balance for each component.
6. Correct statement for a tie component in material balance is
a) Enters a process in only one stream
b) Leaves in only one stream
c) Does not react inside the process
d) All of the mentioned
View Answer
Explanation: A tie component full-fills a, b, c all three conditions.
7-8. For the given drying process, a material (Called SRU) is dried and feed and outputs are as shown in the figure.
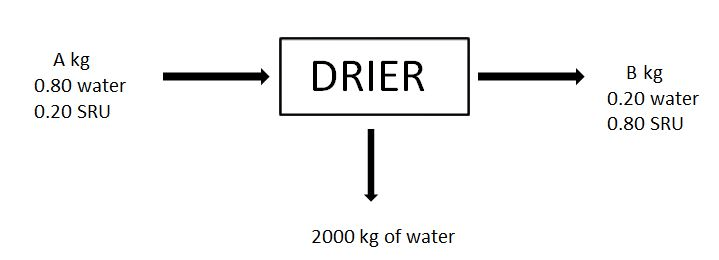
7. A, in kg is
a) 1666.67
b) 2666.67
c) 3666.67
d) 4666.67
View Answer
Explanation: Mass balance for each component.
8. B, in kg is
a) 666.67
b) 566.67
c) 466.67
d) 366.67
View Answer
Explanation: Mass balance for each component.
9-10. A batch of 20% of Acetic acid is prepared by mixing the Acetic acid of two containers A and B. A (400 kg) contains 80% of Acetic acidand B contains 15% of Acetic acid.
9. How much kg of acid is prepared?
a) 5700
b) 7600
c) 8000
d) 9800
View Answer
Explanation: Mass balance for each component.
10. How much B in kg is used?
a) 4000
b) 6000
c) 8000
d) 10000
View Answer
Explanation: Mass balance for each component.
Sanfoundry Global Education & Learning Series – Chemical Process Calculation.
To practice all areas of Chemical Process Calculation, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Check Chemical Process Calculations Books
- Check Chemical Engineering Books
- Practice Chemical Engineering MCQs
- Apply for Chemical Engineering Internship
