This set of Chemical Process Calculation Multiple Choice Questions & Answers (MCQs) focuses on “Enthalpy – I”.
1. Enthalpy (H) is
a) H = U + PV
b) H = U – PV
c) H = U*PV
d) None of the mentioned
Where: U-Internal energy, P- Pressure, V- Volume
View Answer
Explanation: H = U + PV.
2. Specific enthalpy is the function of
a) Temperature and Pressure
b) Temperature and Volume
c) Pressure and Volume
d) None of the mentioned
View Answer
Explanation: H = f(T, P).
3. Cp is the change in enthalpy with respect to temperature at constant _____________
a) Pressure
b) Volume
c) Temperature
d) None of the mentioned
View Answer
Explanation: Cp is the change in enthalpy with respect to temperature at constant Pressure.
4. For ideal gases, Enthalpy and Internal energy is only the function of __________
a) Pressure
b) Volume
c) Temperature
d) None of the mentioned
View Answer
Explanation: For ideal gases, Enthalpy and Internal energy is only the function of Temperature.
5. Enthalpy has no absolute value. Only changes can be calculated.
The given statement is
a) Correct
b) Incorrect
c) Always incorrect
d) None of the mentioned
View Answer
Explanation: Enthalpy is calculated with respect to a reference, there is no absolute value.
6. For ideal gases, the heat capacity relation is
a) Cp = Cv – R
b) Cv = Cp + R
c) Cp – Cv = R
d) None of the mentioned
View Answer
Explanation: Cp – Cv = R.
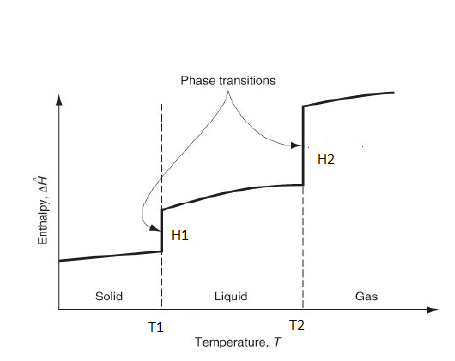
7-10. For the given figure
7. What is T1?
a) T, melting
b) T, freezing
c) T, vaporization
d) T, condensation
View Answer
Explanation: T1 is the melting temperature.
8. What is T2?
a) T, melting
b) T, freezing
c) T, vaporization
d) T, condensation
View Answer
Explanation: T2 is the vaporization temperature.
9. What is H1?
a) Heat of fusion
b) Heat of vaporization
c) Heat of sublimation
d) None of the mentioned
View Answer
Explanation: H1 is the heat of fusion.
10. What is H2?
a) Heat of fusion
b) Heat of vaporization
c) Heat of sublimation
d) None of the mentioned
View Answer
Explanation: H2 is the heat of vaporization.
Sanfoundry Global Education & Learning Series – Chemical Process Calculation.
To practice all areas of Chemical Process Calculation, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Practice Chemical Engineering MCQs
- Apply for Chemical Engineering Internship
- Check Chemical Engineering Books
- Check Chemical Process Calculations Books