This set of Applied Chemistry Multiple Choice Questions & Answers (MCQs) focuses on “Preparation, Properties and Applications of some Compounds – 1”.
1. Which of the following is the incorrect equation?
a) ![]()
b)![]()
c) ![]()
d) 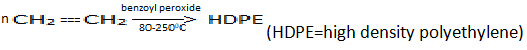
View Answer
Explanation: Ethylene gas in presence of benzoyl peroxide produces the LDPE not HDPE. Here, LDPE is the low density polyethylene. LDPE is produced at 1500 atmosphere pressure.
2. Polyethylene is a ___________
a) Bad conductor
b) Transparent
c) Polar material
d) High symmetrical structure
View Answer
Explanation: Polyethylene is a rigid, waxy white, translucent, non-polar material, with high symmetrical structure. It is a good electrical insulator.
3. Which of the following is attacks the polyethylene?
a) Kerosene
b) Strong acids
c) Alkalis
d) Salt solutions
View Answer
Explanation: The polyethylene is resistant to strong acids, alkalis and salt solutions. It is attacked by the oils and organic solvents. It is also resistant to oxygen, carbondioxide.
4. The low density polyethylene has a melting point.
a) 840C
b) 850C
c) 860C
d) 870C
View Answer
Explanation: The low density polyethylene possess branched chain structure and its melting point is about the 870C.
5. Poly vinyl chloride is produced by the free radical chain polymerisation of the vinyl chloride in presence of the benzoyl peroxide.
a) True
b) False
View Answer
Explanation: Poly vinyl chloride is produced by the free radical chain polymerisation of the vinyl chloride in presence of the benzoyl peroxide. The hydrogen peroxide can also be used as the catalyst instead of the benzoyl peroxide.
6. Poly vinyl chloride is a __________
a) Blue coloured compound
b) Inflammable
c) Weak
d) Brittle
View Answer
Explanation: Poly vinyl chloride is a colourless, non-inflammable and chemically inert in nature. It is strong and brittle.
7. Plasticized poly vinyl chloride can be used for _________
a) High frequency insulator parts
b) Bottle caps
c) Coated wires
d) Electrical insulation
View Answer
Explanation: Plasticized poly vinyl chloride can be used for electrical insulation, injection moulding articles like tool handles, radio and telephone components.
8. Phenol is made to react with formaldehyde in the presence of acid or alkali produces ________
a) Phenol
b) Poly vinyl chloride
c) Plasticized poly vinyl chloride
d) Polyethylene
View Answer
Explanation: Phenol is made to react with formaldehyde in the presence of acid or alkali produces di, tri and mono phenols depending on the phenol formaldehyde ratio.
9. Bakelite is __________
a) Good anion exchanging resin
b) Attacked by acids
c) Attacked by salts
d) Resistant to alkalis
View Answer
Explanation: Bakelite is a good anionic exchanging resin. It is a good adhesive and it is resistant to acids and salts. It is attacked by the alkalis.
10. Glass laminates can be made by using ___________
a) Poly vinyl chloride
b) Bakelite
c) Polyethylene
d) Phenol
View Answer
Explanation: Glass laminates can be made by using Bakelite. Bakelite is also called as the phenol-formaldehyde resin.
11. Bakelite is not _______
a) Hard
b) Strong
c) Rigid
d) Weak
View Answer
Explanation: Bakelite is not weak. It is hard, strong and rigid. It is an excellent electrical insulator. It is scratch resistant and water resistant.
12. The bearings used in the propeller shafts are prepared using ________
a) Phenol-formaldehyde resin
b) TEFLON
c) Vinyl cyanide
d) Vinyl iso cyanide
View Answer
Explanation: The bearings used in the propeller shafts are made by using Phenol-formaldehyde resin. It is also used in the paper industry and rolling mills.
13. TEFLON is obtained by the chain polymerisation of tetra fluoro ethylene in presence of __________ as initiator.
a) Hydrogen peroxide
b) Hydrogen nitrate
c) Hydrogen
d) Benzoyl peroxide
View Answer
Explanation: TEFLON is obtained by the chain polymerisation of the tetra fluoro ethylene in presence of the benzoyl peroxide as an initiator.
14. TEFLON has _________
a) High melting point
b) Low melting point
c) Low density
d) Good conduction of electricity
View Answer
Explanation: TEFLON has a high melting point, high density and it is the bad conductor of electricity as it is an insulator.
15. TEFLON is used to make chemical carry pipes due to its __________
a) extreme chemical resistance
b) Resistance towards alkalis
c) Resistance towards strong acids
d) Resistance towards salts
View Answer
Explanation: TEFLON is used to make chemical carry pipes due to its extreme chemical resistance. It is used for making the gaskets, pump parts, tank linings and tubing.
Sanfoundry Global Education & Learning Series – Applied Chemistry.
To practice all areas of Applied Chemistry, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
