This set of Analytical Instrumentation Multiple Choice Questions & Answers (MCQs) focuses on “NO2 Analyser”.
1. Nitrogen dioxide can be detected with a paramagnetic analyser.
a) True
b) False
View Answer
Explanation: Nitrogen dioxide can be detected with a paramagnetic analyser. This is because nitrogen has paramagnetic property ie. it is attracted by magnetic fields.
2. Nitrogen oxide cannot be directly analysed using UV and Visible analyzers due to which of the following reasons?
a) Less accuracy
b) Very low range
c) It leads to contamination of the sample
d) It is transparent in UV visible regions
View Answer
Explanation: Nitrogen oxide cannot be directly analysed using UV and Visible analyzers because it is transparent in UV visible regions. Hence, it is converted into nitrogen dioxide and then analysed.
3. How is NO converted to NO2 for analysis in UV and Visible analyzers?
a) Treating sample gas with pressurized oxygen
b) Treating sample gas with ozone
c) Treating sample gas with oxygen at low pressure
d) Treating sample gas with water at high pressure
View Answer
Explanation: NO is converted into NO2 for analysis in UV and Visible analyzers by treating sample gas with pressurized oxygen. This is because direct measurement of NO is not possible using UV and Visible analyzers.
4. How is NO converted to NO2 for analysis in Chemiluminescent analyser?
a) Treating sample gas with pressurized oxygen
b) Treating sample gas with ozone
c) Treating sample gas with oxygen at low pressure
d) Treating sample gas with water at high pressure
View Answer
Explanation: NO is converted to NO2 for analysis in Chemiluminescent analyser by treating sample gas with ozone.
NO + O3 —>NO2 + O2
5. During analysis of NO2 using Chemiluminescent analyser, why is NO2 not made to react with ozone directly?
a) Less accuracy
b) It is a slow process
c) It leads to contamination of the sample
d) It does not produce luminescence
View Answer
Explanation: During analysis of NO2 using Chemiluminescent analyser, NO2 is not made to react with ozone directly because it is a slow process. Hence, it is converted to NO using catalytic reactions or converters.
6. The block diagram of series mode analyser of NO and NO2 is given below. Identify the unmarked procedure.
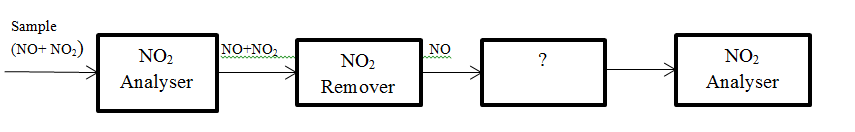
a) Oxidiser
b) Reducer
c) Filter
d) NO Analyser
View Answer
Explanation: The unmarked procedure is NO to NO2 oxidiser. NO2 is then analysed by NO2 analyser.
7. The block diagram for parallel mode analyser of NO and NO2 is given below. Identify the unmarked block in the diagram.
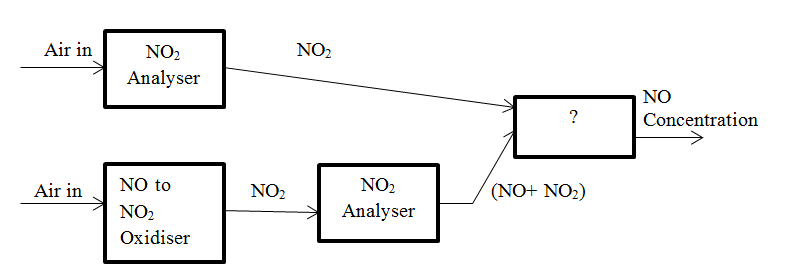
a) NO Analyser
b) NO2 to NO reduction
c) NO2 remover
d) Difference
View Answer
Explanation: The unmarked block is Difference. Difference between the concentration of NO2 and NO and the concentration of NO2 alone is determined and the result gives the concentration of NO.
8. The instruments based on chemiluminescence maintain linearity in which of the following ranges?
a) 1ppb to 100ppb
b) 100ppb to 1000ppb
c) 1ppb to 1000ppb
d) 100ppb to 1000ppb
View Answer
Explanation: The instruments based on chemiluminescence maintain linearity between 1ppb to 1000ppb. These are used to measure NO in exhaust gases in vehicles.
9. How can absorption be enhanced while determining NO concentration using CO laser?
a) By converting NO into NO2
b) By placing NO in a magnetic field
c) By using proper monochromators
d) By using choppers
View Answer
Explanation: CO laser emits radiation that can absorb NO. Absorption is enhanced by placing NO in a magnetic field.
10. Which of the following detectors are generally used for detection in NO analysis using CO laser?
a) Photomultiplier tube
b) Photovoltaic cell
c) Liquid nitrogen cooled Ge-Au element
d) Photo emissive tube
View Answer
Explanation: Liquid nitrogen cooled Ge-Au element is used detection in NO analysis using CO laser. The signal amplitude varied with the concentration of NO.
11. Which of the following analyzers are used to measure trace amounts of nitrogen oxides in the stratosphere?
a) Chemiluminescence
b) CO laser method
c) Laser opto-acoustic spectroscopy
d) Colorimetry
View Answer
Explanation: Laser opto-acoustic spectroscopy is used to measure trace amounts of nitrogen oxides in the stratosphere. It was developed for application in air pollution measurement.
12. A pink coloured dye complex is formed when air containing NO2 is passed in an absorbing solution consisting of __________ and diamine dissolved in the acetic acid medium.
a) Sulphuric acid
b) Sulphonyl
c) Sulphonic acid
d) Sulphanilic acid
View Answer
Explanation: A pink coloured dye complex is formed when air containing NO2 is passed in an absorbing solution consisting of sulphanilic acid and diamine dissolved in the acetic acid medium. This is called Saltzman method.
13. In Laser Opto-acoustic spectroscopy, the IR beam excites the molecules to higher states. In which of the following ways do the molecules return to the ground state?
a) Collisional de-excitation
b) Random de-excitation
c) By spontaneous emission
d) By stimulated emission
View Answer
Explanation: The molecules return to the ground state by collisional de-excitation. This results in an increase in temperature.
14. To monitor oxides of nitrogen in stack effluents, the sample containing oxides of nitrogen is passed through a flask containing solution of H2O2 in sulphuric acid. Nitric acid is formed. The nitrate ions then react with phenol-disulphonic acid to produce blue colour.
a)True
b) False
View Answer
Explanation: The nitrate ions react with phenol-disulphonic acid to produce yellow colour. This is measured colorimetrically.
Sanfoundry Global Education & Learning Series – Analytical Instrumentation.
To practice all areas of Analytical Instrumentation, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Check Analytical Instrumentation Books
- Apply for Instrumentation Engineering Internship
- Practice Instrumentation Engineering MCQs
- Check Instrumentation Engineering Books
