This set of Analytical Instrumentation Multiple Choice Questions & Answers (MCQs) focuses on “Hydrogen and Gas Electrodes”.
1. Which of the following is not the characteristic of a reference electrode?
a) It must have a known output potential
b) It must have a constant output potential
c) Its output potential is dependent on the composition of the solution
d) It is employed in conjunction with the indicator or working electrode
View Answer
Explanation: The output potential of a reference electrode must be insensitive to the composition of the solution.
2. Why is Standard hydrogen electrode called as the primary reference electrode?
a) It has a known output potential
b) It has a constant output potential
c) Its output potential is independent of the composition of the solution
d) Its output potential is zero volts
View Answer
Explanation: Standard hydrogen electrode is called the primary reference electrode as its output potential is zero volts. It is employed in conjunction with the indicator or working electrode.
3. Which of the following is the simple and most convenient hydrogen electrode?
a) Pascal Hydrogen electrode
b) Bourne Hydrogen electrode
c) Hilderbant Hydrogen electrode
d) West Hydrogen electrode
View Answer
Explanation: The hydrogen electrode given by Hilderbant is the simple and most convenient hydrogen electrode. A number of hydrogen electrodes are available.
4. Which of the following is not the disadvantage of hydrogen electrode?
a) Platinum can be easily poisoned
b) Presence of oxidising agents alters the potential
c) It gives a salt error
d) H2 gas at 1 atmospheric pressure is difficult to set up and transport
View Answer
Explanation: Hydrogen electrode does not give a salt error. A number of hydrogen electrodes are available.
5. In Hydrogen electrode, the electrode is placed in a solution of ____ M Hcl. Fill in the blank.
a) 0.5
b) 1
c) 2
d) 3
View Answer
Explanation: In Hydrogen electrode, the electrode is placed in a solution of 1M Hcl. H2 gas at 1 atm pressure is passed through the side arm in such a way that the platinum is half immersed in Hcl.
6. Hydrogen electrode which is the reference electrode can be used as which of the following?
a) Anode only
b) Cathode only
c) Anode or Cathode
d) Salt bridge
View Answer
Explanation: Hydrogen electrode which is the reference electrode can be used as the anode or the cathode. It depends on the half-cell to which it is coupled.
7. If hydrogen electrode acts as cathode, hydrogen is reduced.
a) True
b) False
View Answer
Explanation: If hydrogen electrode acts as cathode, hydrogen is reduced. If hydrogen electrode acts as anode, hydrogen is oxidised.
8. Given below is a diagram of hydrogen electrode. Identify the unmarked component.
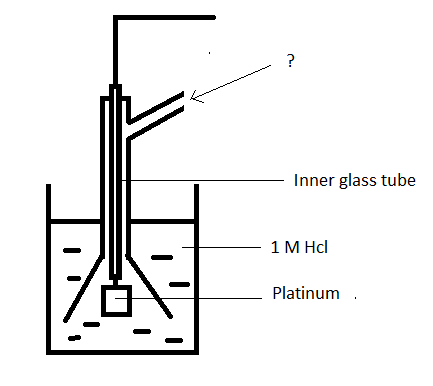
a) Hydrogen at 1 atm
b) Hydrogen at 10 atm
c) Helium at 1 atm
d) Helium at 10 atm
View Answer
Explanation: Hydrogen at 1 atm is sent through the side tube. The electrode is placed in a solution of 1M Hcl.
9. The composition of glass membrane in glass electrode cannot have which of the following?
a) Sodium silicate
b) Calcium silicate
c) Lithium silicate
d) Barium silicate
View Answer
Explanation: Glass electrode consists of either sodium or calcium silicate or lithium silicates containing glass membrane. It has lanthanum and barium ions added to the membrane.
10. Which of the following is the purpose of added membranes in the glass membrane of the glass electrode?
a) They act as tightners
b) They act as filters
c) They act as conditioners
d) They act as collectors
View Answer
Explanation: The ions in the added membranes act as tightners. They reduce the mobility of sodium ion.
11. Which of the following cannot form the inner reference electrode in glass electrodes?
a) Silver electrode
b) Copper electrode
c) Calomel electrode
d) Silver chloride electrode
View Answer
Explanation: Copper electrode cannot form the inner reference electrode in glass electrodes. Inner reference electrode is immersed in a buffer solution.
12. The pH response of glass electrode is limited entirely to the area of the special glass membrane bulb.
a) True
b) False
View Answer
Explanation: The pH response of glass electrode is limited entirely to the area of the special glass membrane bulb. The response of the electrode is independent of the depth of immersion.
13. Which of the following is not the advantage of glass electrodes?
a) It gives accurate results for high as well as low pH values
b) It is simple to operate
c) It has no salt error
d) Modern electrodes can withstand severe treatment
View Answer
Explanation: It gives accurate results for low pH values only ie. from 0 to 9. For high pH values, the glass becomes responsive to sodium and other cations.
14. Which of the following is not the disadvantage of glass electrodes?
a) Poor readings are obtained in buffered or unbuffered solutions
b) The electrode must be washed thoroughly with distilled water to obtain proper results
c) Materials suspended on glass should be wiped out neatly to obtain proper results
d) It is affected by oxidation reduction potentials in the solution
View Answer
Explanation: It is affected by oxidation reduction potentials in the solution. It is an advantage of the glass electrode.
15. Given below is the diagram of glass electrode. Identify the unmarked component.
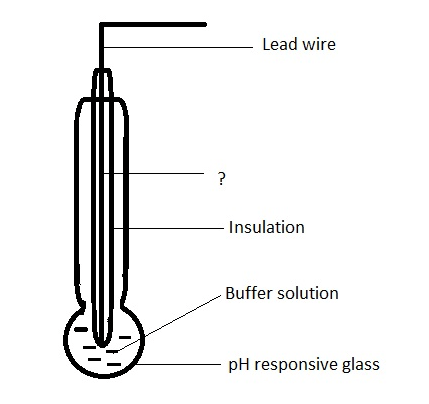
a) Platinum leads
b) Silver wire coated with silver chloride
c) Copper wire
d) Platinum reference electrode
View Answer
Explanation: The unmarked component is silver wire coated with silver chloride. It forms the inner reference electrode.
Sanfoundry Global Education & Learning Series – Analytical Instrumentation.
To practice all areas of Analytical Instrumentation, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Check Instrumentation Engineering Books
- Apply for Instrumentation Engineering Internship
- Check Analytical Instrumentation Books
- Practice Instrumentation Engineering MCQs
