This set of Thermodynamics Multiple Choice Questions & Answers (MCQs) focuses on “Second Law of Thermodynamics”.
1. Heat is transferred to a heat engine from a furnace at a rate of 80 MW. If the rate of waste heat rejection to a nearby river is 50 MW, determine the net power output for this heat engine.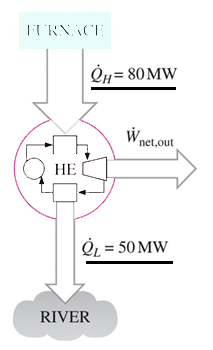
a) 30 MW
b) 40 MW
c) 50 MW
d) 60 MW
View Answer
Explanation: Net power output = 80 – 50 MW = 30 MW.
2. Heat is transferred to a heat engine from a furnace at a rate of 80 MW. If the rate of waste heat rejection to a nearby river is 50 MW, determine the thermal efficiency for this heat engine.
a) 47.5 %
b) 27.5 %
c) 37.5 %
d) none of the mentioned
View Answer
Explanation: The thermal efficiency of heat engine = net work output / heat input
= 30/80 = 0.375 = 37.5 %.
3. A car engine with a power output of 50 kW has a thermal efficiency of 24 percent. Determine the fuel consumption rate of this car if the fuel has a heating value of 44,000 kJ/kg . 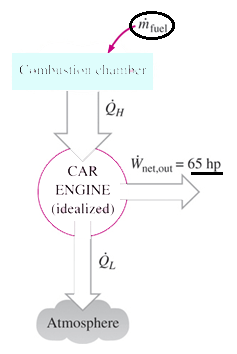
a) 0.00273 kg/s
b) 0.00373 kg/s
c) 0.00473 kg/s
d) 0.00573 kg/s
View Answer
Explanation: Q = 50/0.24 = 208.3 kW,
hence fuel consumption rate = 208.3 kW / 44000 kJ/kg = 0.00473 kg/s.
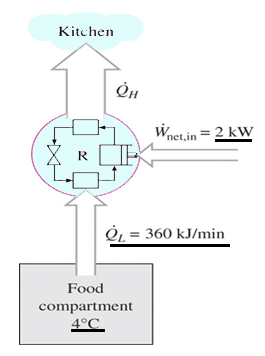
4. The food compartment of a refrigerator is maintained at 4°C by removing heat from it at a rate of 360 kJ/min. If the required power input to the refrigerator is 2kW, determine the coefficient of performance of the refrigerator.
a) 4
b) 3
c) 2
d) 1
View Answer
Explanation: COP = (360/2)(1/60) = 3.
5. The food compartment of a refrigerator is maintained at 4°C by removing heat from it at a rate of 360 kJ/min. If the required power input to the refrigerator is 2kW, determine the rate of heat rejection to the room that houses the refrigerator.
a) 450 kJ/min
b) 460 kJ/min
c) 470 kJ/min
d) 480 kJ/min
View Answer
Explanation: Q = 360 + (2)(60/1) = 480 kJ/min.
6. A heat pump is used to meet the heating requirements of a house and maintain it at 20°C. On a day when the outdoor air temperature drops to 2°C, the house is estimated to lose heat at a rate of 80,000 kJ/h. If the heat pump under these conditions has a COP of 2.5, determine the power consumed by the heat pump.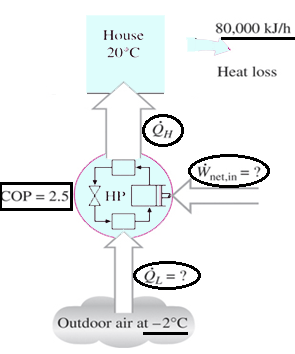
a) 32000 kJ/h
b) 33000 kJ/h
c) 34000 kJ/h
d) 35000 kJ/h
View Answer
Explanation: W = Q/COP = 80000 kJ/h / 2.5 = 32000 kJ/h.
7. A heat pump is used to meet the heating requirements of a house and maintain it at 20°C. On a day when the outdoor air temperature drops to 2°C, the house is estimated to lose heat at a rate of 80,000 kJ/h. If the heat pump under these conditions has a COP of 2.5, determine the rate at which heat is absorbed from the cold outdoor air.
a) 32000 kJ/h
b) 48000 kJ/h
c) 54000 kJ/h
d) 72000 kJ/h
View Answer
Explanation: The rate at which heat is absorbed = 80000 – 32000 = 48000 kJ/h.
8. An air-conditioner provides 1 kg/s of air at 15°C cooled from outside atmospheric air at 35°C. Estimate the amount of power needed to operate the air-conditioner.
a) 1.09 kW
b) 1.19 kW
c) 1.29 kW
d) 1.39 kW
View Answer
Explanation: Q = m*cp*(temperature change) = 20.08 kW
COP = (15+273)/(35-15) = 14.4
hence power needed = 20/14.4 = 1.39 kW.
9. A cyclic machine, as shown below, receives 325 kJ from a 1000 K energy reservoir. It rejects 125 kJ to a 400 K energy reservoir and the cycle produces 200kJ of work as output. Is this cycle reversible, irreversible, or impossible?
a) reversible
b) irreversible
c) impossible
d) none of the mentioned
View Answer
Explanation: The Carnot efficiency = 1 – (400/1000) = 0.6 and real efficiency = (300/325) = 0.615 which is greater than the Carnot efficiency hence cycle is impossible.
10. In a cryogenic experiment you need to keep a container at -125°C although it gains 100 W due to heat transfer. What is the smallest motor you would need for a heat pump absorbing heat from the container and rejecting heat to the room at 20°C?
a) 97.84 kW
b) 98.84 kW
c) 99.84 kW
d) 95.84 kW
View Answer
Explanation: COP = 1.022 and thus power required = 100/1.022 = 97.84 kW.
11. A car engine operates with a thermal efficiency of 35%. Assume the air-conditioner has a coefficient of performance of 3 working as a refrigerator cooling the inside using engine shaft work to drive it. How much fuel energy should be spend extra to remove 1 kJ from the inside?
a) 0.752 kJ
b) 0.952 kJ
c) 0.852 kJ
d) none of the mentioned
View Answer
Explanation: W = thermal efficiency * Q(fuel) thus Q(fuel) = 1/(0.35*3) = 0.952 kJ.
Sanfoundry Global Education & Learning Series – Thermodynamics.
To practice all areas of Thermodynamics, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Check Chemical Engineering Books
- Practice Mechanical Engineering MCQs
- Check Thermodynamics Books
- Apply for Chemical Engineering Internship
- Apply for Mechanical Engineering Internship
