This set of Pulp and Paper Multiple Choice Questions and Answers (MCQs) focuses on “Surface Tension and Sizing”.
1. Identify the equation used to calculate pore sizes.
l2 = rty cosθ/2ŋ
a) Lucas
b) Wettability
c) Kelvin
d) Pascal
View Answer
Explanation: Lucas used this equation to calculate pore sizes in paper based on the speed of the rise of organic liquids in paper strips. Lucas used organic liquids since they would not change the pore sizes by swelling or hydration.
2. Identify the equation to give actual capillary action.
Pα – Pβ= 2y / R
a) Lucas
b) Wettability
c) Kelvin
d) Pascal
View Answer
Explanation: This equation is modified to give the actual capillary rise for H2O by solving for the force of H2O and the wettability of the glass by the H2O. If the H2O completely wets the glass. Here, r is the capillary tube radius, p is the density of a phase, g is the acc. due to gravity, and h is the height of the H2O.
3. Identify the equation to give actual capillary action.
y = 1/2(ρα – ρβ)ghr / cosθ
a) Lucas
b) Wettability
c) Kelvin
d) Pascal
View Answer
Explanation: This equation is modified to give the actual capillary rise for H2O by solving for the force of H2O and the wettability of the glass by the H2O. If the H2O completely wets the glass. Here, r is the capillary tube radius, p is the density of a phase, g is the acc. due to gravity, and h is the height of the H2O.
4. 3 contact angles of a droplet on a flat surface
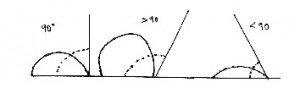
a) True
b) False
View Answer
Explanation: Either at 90 degrees or an acute angle or an obtuse angle. Contact angel is the angle, normally measured through the liquid, where a liquid–vapor interface contacts a solid surface.
5. Find the hidden term
if adhesive > cohesive then 0 degree <= θ <_________
a) 10 degrees
b) 180 degrees
c) 100 degrees
d) 90 degrees
View Answer
Explanation: The contact angle is a measure of the adhesive force of the liquid with the surface relative to the cohesive forces in the liquid. It is generally measured through the liquid, where a liquid–vapour interface contacts a solid surface.
6. Find the hidden term
if adhesive > cohesive then 90 degree <= θ <_________
a) 10 degrees
b) 180 degrees
c) 100 degrees
d) 90 degrees
View Answer
Explanation: The contact angle is a measure of the adhesive force of the liquid with the surface relative to the cohesive forces in the liquid. The angle, conventionally measured through the liquid, where a liquid–vapour interface contacts a solid surface.
7. _________ is easily wetted by H2O and, H2O on _________ gives a low contact angle that approaches zero; this causes the water to be drawn into the capillaries. [/expand]
a) Starch
b) Lignin
c) Cellulose
d) Pulp
View Answer
Explanation: Cellulose is easily wetted by H2O and, H2O on cellulose gives a low contact angle that approaches zero; this causes the H2O to be drawn into the capillaries.
8. Internal sizing agents are generally _________ materials. The polar portion attaches to the fiber or fines directly through a covalent bond or indirectly through a caustic element such as alum.
a) Amphoteric
b) Amphitaric
c) Non polar
d) Polar
View Answer
Explanation: Internal sizing agents are generally amphipathic having polar and nonpolar moieties on the same molecule materials. The polar portion more importantly, it is chemically reactive attaches to the fiber or fines directly through a covalent bond.
9. Identify the equation used to calculate pore radius from which water will condense.
r = (2yV) / RT ln (po/p)
a) Lucas
b) Wettability
c) Kelvin
d) Pascal
View Answer
Explanation: A water droplet with a (+ve) radius has a slightly higher vapor pressure than a flat surface of H2O. When with a (-ve) radius has a lower vapor pressure, which allows condensation to occur.
10. Inverse _________has been utilized to characterize surface energy; in pulp and paper this has been accomplished with treated and untreated pulps.
a) Gas chromatography
b) Silver test
c) Stone test
d) Sulfurication
View Answer
Explanation: It’s a common type of chromatography utilized in analytical chemisty for separating and analyzing compounds that could be vaporized without decomposition. Typical uses of G.C. include testing the purity of a particular substance, or separating the different components of a mixture.
Sanfoundry Global Education & Learning Series – Pulp and Paper.
To practice all areas of Pulp and Paper, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Apply for Chemical Engineering Internship
- Practice Chemical Engineering MCQs
- Check Chemical Engineering Books
