This set of Pulp and Paper Multiple Choice Questions and Answers (MCQs) focuses on “Selected Reactions of Carbohydrates”.
1. Acid hydrolysis becomes important in the pulp and paper industry whenever we process wood fibers below pH 2 or so at elevated temperatures.
a) True
b) False
View Answer
Explanation: Acid hydrolysis becomes important in the pulp and paper industry whenever we process wood fibers below pH 2 or so at elevated temperatures.
2. If one treats _________ with 6 % w/w aqueous sulfuric acid under reflux, glucose is obtained in high yield with little secondary decomposition.
a) Cellulose
b) Fructose
c) Starch
d) Lignin
View Answer
Explanation: If one treats cellulose (which has been swelled in 72 % w/w sulfuric acid at room temperature) with 6 % w/w aqueous sulfuric acid under reflux, glucose is obtained in high yield with little secondary decomposition.
3. If _________ are treated with strong acid such as 20% sulfuric acid at high temperatures, they are first hydrolyzed to the component _________
a) Polysaccharides, monosaccharides
b) Monosaccharides, polysaccharides
c) Fructose, cellulose
d) Lignin, pulp
View Answer
Explanation: If polysaccharides are treated with strong acid such as 20% sulfuric acid at high temperatures, they are first hydrolyzed to the component monosaccharides.
4. One can reduce the reducing end of mono- or polysaccharides by using sodium borohydride (NaBH4) under _________ conditions at room temperature.
a) Acidic
b) Basic
c) Alkaline
d) Neutral
View Answer
Explanation: One can reduce the reducing end of mono- or polysaccharides by using sodium borohydride (NaBH4) under alkaline conditions at room temperature.
5. Under conditions of dilute alkali (perhaps 0.1 M NaOH at 100°C) _________ will slowly undergo a reaction that causes C-2 epimerization (a change in configuration of the second carbon atom).
a) Polymers
b) Polysaccharides
c) Monosaccharides
d) Fructose
View Answer
Explanation: Under conditions of dilute alkali (perhaps 0.1 M NaOH at 100°C) monosaccharides (and the reducing group of polysaccharides) will slowly undergo a reaction that causes C-2 epimerization (a change in configuration of the second carbon atom).
6. What is the name of the question mark area?
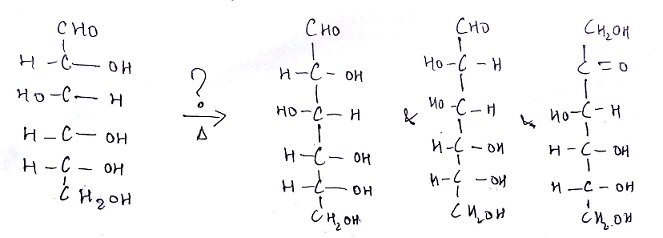
a) 25% H2SO4, Reflux
b) 6% H2SO4, Reflux
c) Dilute OH-
d) Maximum Heat
View Answer
Explanation: Under conditions of dilute alkali (perhaps 0.1 M NaOH at 100°C) monosaccharides (and the reducing group of polysaccharides) will slowly undergo a reaction that causes C-2 epimerization (a change in configuration of the second carbon atom)
7. What is the name of the question mark area?
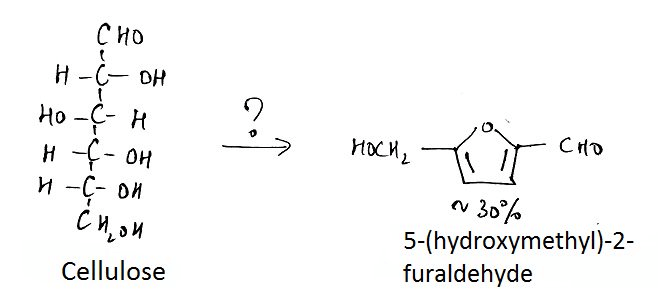
a) 25% H2SO4, Reflux
b) 6% H2SO4, Reflux
c) Dilute OH-
d) Maximum Heat
View Answer
Explanation: The reaction of hexoses, or polymers of hexoses such as cellulose, starch, and glucomannans, produces 5- (hydroxymethyl)-2-fiirfiiral that is not volatile and undergoes decomposition to levulinic acid and numerous other compounds.
8. What is the name of the question mark area?
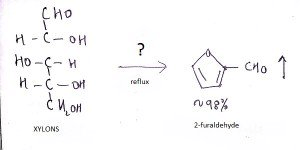
a) 25% H2SO4, Reflux
b) 6% H2SO4, Reflux
c) Dilute OH-
d) Maximum Heat
View Answer
Explanation: The reaction of hexoses, or polymers of hexoses such as cellulose, starch, and glucomannans, produces 5- (hydroxymethyl)-2-fiirfiiral that is not volatile and undergoes decomposition to levulinic acid and numerous other compounds.
9. What is the name of the question mark area?
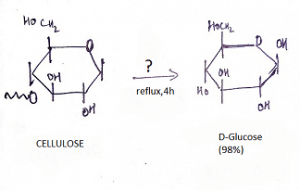
a) 25% H2SO4, Reflux
b) 6% H2SO4, Reflux
c) Dilute OH-
d) Maximum Heat
View Answer
Explanation: If one treats cellulose (which has been swelled in 72 % w/w sulfuric acid at room temperature) with 6 % w/w aqueous sulfuric acid under reflux, glucose is obtained in high yield with little secondary decomposition.
10. What is the name of the question mark area?
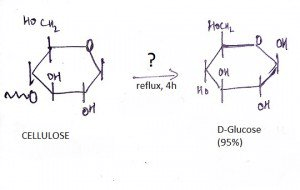
a) 25% H2SO4, Reflux
b) 6% H2SO4, Reflux
c) Dilute OH-
d) Maximum Heat
View Answer
Explanation: If one treats cellulose (which has been swelled in 72 % w/w sulfuric acid at room temperature) with 6 % w/w aqueous sulfuric acid under reflux, glucose is obtained in high yield with little secondary decomposition.
Sanfoundry Global Education & Learning Series – Pulp and Paper.
To practice all areas of Pulp and Paper, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Check Chemical Engineering Books
- Practice Chemical Engineering MCQs
- Apply for Chemical Engineering Internship
