This set of Polymer Engineering Multiple Choice Questions & Answers (MCQs) focuses on “Characteristics of Condensation Polymerization”.
1. Consider a reaction of polymer formation by condensation polymerization, completed in n-steps, with the liberation of a certain byproduct. How many total molecules of byproduct are released as a result of complete reaction?
a) n+1
b) n
c) n-1
d) n/2
View Answer
Explanation: At each step of a condensation polymerization reaction, one byproduct molecule is released. So a total of n molecules are liberated.
2. How many molecules of hydrochloric acid are released when n monomers of di-acid chloride and n monomers of di-alcohol are reacted to form a polymer?
a) 2n
b) n-1
c) 2n-1
d) n
View Answer
Explanation: Here, the reaction between the monomers completes in (2n-1) number of steps. So, the molecules released will also be 2n-1.
3. Which of the byproduct molecule is released in the formation of polyurea?
a) NH3
b) H2O
c) HCl
d) no elimination
View Answer
Explanation: There is no byproduct elimination in this reaction.
Formation of urea-
─N═C═O + H2N─ ↔ ─NHCO─NH─
Formation of polyurea-
nOCRNCO + n H2NR’NH2 ↔ OCN─[─RNHCO─HNR’NHCONH]n-1─RNHCONHR’NH2.
4. Which of the following byproduct is released when an amine reacts with acid chloride?
a) HCl
b) H2O
c) NH3
d) no elimination
View Answer
Explanation: The amidation reaction is given by-
─NH2 + ─COCl ↔ ─NH─CO─ + HCl.
5. Which of the following byproduct is released when an ester reacts with alcohol?
a) alcohol
b) water
c) ether
d) no elimination
View Answer
Explanation: The reaction is given by-
─COOR1 + R2OH ↔ ─COOR2 + R1OH
Thus, the byproduct is alcohol.
6. Which of the following reactants react to give amidation reaction?
a) amine and acid
b) amine and alcohol
c) amine and ester
d) ester and alcohol
View Answer
Explanation: The reaction of amine with acid is an amidation reaction which is given by-
─NH2 + ─COOH ↔ ─NH─CO─ + H2O.
7. Which of the following does not result in the formation of an ester?
a) acid and alcohol
b) acid chloride and alcohol
c) ester and alcohol
d) amine and acid
View Answer
Explanation: The reaction of acid and acid chloride with alcohol is an esterification reaction, and even the reaction of ester and alcohol give rise to formation of ester. Amine and acid react to give amide group.
8. Consider the following statements for condensation polymerization-
I. Bifunctional or polyfunctional monomers
II. Loss of each kind of functional group in each step for bifunctional species
III. Always accompanied by the release of a byproduct molecule
IV. Monofunctional or polyfunctional monomers
Which of the following are true?
a) I and II
b) I, II and III
c) I and III
d) III and IV
View Answer
Explanation: III is not true because in some reactions, there is no elimination of any molecule. Besides, monofuntional monomers fail to complete the reaction. At each step of reaction, two functional groups, one of each kind, are lost.
9. What is the product of following polymerization reaction?
![The product of following polymerization reaction is [─O─R─CO─]n](https://www.sanfoundry.com/wp-content/uploads/2017/05/polymer-engineering-questions-answers-characteristics-condensation-polymerisation-q9.png) ↔
↔
a) [─R─CO─]
b) [─O─R─CO─]n
c) [O─CR─O─]n
d) [─O─R─CO─]n-1
View Answer
Explanation: The lactone ring opens by breaking C─O bond and polymerizes to give [─O─R─CO─]n.
10. What is the product of following polymerization reaction?
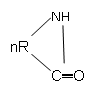
a) ─[─NH─R─CO─]n
b) ─[─R─NH─CO─]n
c) ─[─NH─R─CO─]n-1
d) none of the mentioned
View Answer
Explanation: The lactam group polymerizes to give ─[─NH─R─CO─]n product, consisting of amide group.
Sanfoundry Global Education & Learning Series – Polymer Engineering.
To practice all areas of Polymer Engineering, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Practice Chemical Engineering MCQs
- Check Polymer Technology Books
- Apply for Chemical Engineering Internship
- Check Chemical Engineering Books
