This set of Mass Transfer Multiple Choice Questions & Answers (MCQs) focuses on “Interphase Mass Transfer”.
1. The Concentration of the two phases in a closed system at the Interphase is
a) Changes continuously
b) Never changes
c) Becomes zero
d) Increases till the driving force becomes zero
View Answer
Explanation: The concentration changes only if the component of the two phases added or removed. Generally, Interphase occurs at equilibrium. Once the additional component is added to a system at equilibrium, the concentration changes till it become uniform but it will be differ from the previous.
2. Diffusion of components between the phases at equilibrium is
a) Zero
b) Infinity
c) Changes continuously
d) Diffusion never occurs
View Answer
Explanation: At equilibrium, the concentration becomes uniform so the rate of diffusion stops.
3. Consider a steady-state condition; the concentration at any point in the equipment never changes with time.
a) True
b) False
View Answer
Explanation: Generally at a steady state, the net transfer remains same so the concentration will be same at every point.
4. Find the x & y co-ordinate in the figure below at steady state. Here, the gas phase and liquid phase mole fraction representation is given below
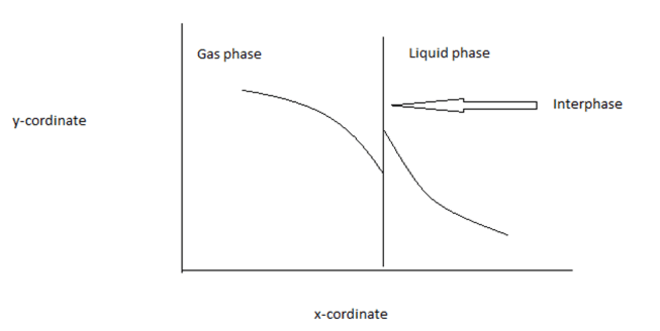
a) x- distance & y- concentration
b) x-time & y- concentration
c) x-concentration & y- time
d) x-concentration & y-distance
View Answer
Explanation: Generally, the concentration changes with distance but never changes with time at steady state. From the concept of driving force, the y- coordinate should be concentration.
5. The real driving force of the mass transfer is
a) Chemical potential
b) Physical potential
c) Pressure gradient
d) Concentration gradient
View Answer
Explanation: Chemical potential represents the dynamic equilibrium of the mass transfer.
6. According to Lewis and Whitman theory, the departure from concentration equilibrium at the Interphase is due to
a) Low mass transfer rates
b) High mass transfer rates
c) Moderate mass transfer rate
d) None of the mentioned
View Answer
Explanation: Theoretically proved by Lewis and Whitman, that if the mass transfer rates are higher the concentration deviates from equilibrium.
7. At the Interphase, the Interphase concentration of the component in both phase phases have equal chemical potential is due to differential concentration.
a) True
b) False
View Answer
Explanation: The concentration rise at the Interphase is not a barrier to the diffusion. They are equilibrium concentration which is similar to equal chemical potential.
8. The equilibrium concentrations in the gas and the liquid phases, in mole fraction, give rise to a curve known as
a) Equilibrium distribution curve
b) Equilibrium concentration curve
c) Differential distribution curve
d) Differential concentration curve
View Answer
Explanation: The equilibrium distribution curve represents the phase-phase equilibrium curve with the coordinates of mole fractions in both the phases.
Sanfoundry Global Education & Learning Series – Mass Transfer.
To practice all areas of Mass Transfer, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Check Mass Transfer Books
- Check Mechanical Engineering Books
- Apply for Chemical Engineering Internship
- Check Chemical Engineering Books
- Practice Chemical Engineering MCQs
