This set of Mass Transfer Question Bank focuses on “Fractionation Column-Ponchon & Savarit Method”
1. The Ponchon Savorit method is applicable for determining negligible heat loss.
a) True
b) False
View Answer
Explanation: As this method includes enthalpy concentration data it helps to determine the negligible heat loss.
2. External reflux ratio is used for Ponchon Savorit method.
a) True
b) False
View Answer
Explanation: Here the reflux ratio is based on the flow rates of the final plates of the enriching section or stripping section; so internal reflux ratio comes to the part.
3. Find the heat removed by the condenser if the net heat out of the condenser is 25000 J and the total moles are 25.
a) 1000 J
b) 10000 J
c) 5000 J
d) 2000 J
View Answer
Explanation: Heat removed by the condenser = net heat out/ net moles out
= 25000 J/ 25
= 1000.
4. From the enthalpy-concentration diagram find the location of heat removed by the condenser.

a) X
b) Y
c) Z
d) None of the mentioned
View Answer
Explanation: X is the heat removed by the condenser where the net heat removed by the net moles out can be determined.
5. Find the internal reflux ratio by analysing the below fractionator.
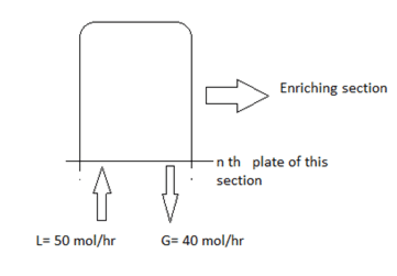
a) 0.8
b) 1.25
c) 1.2
d) 1
View Answer
Explanation: Internal reflux ratio = 50/40 = 1.25.
6. Estimate the heat removed by the condenser.
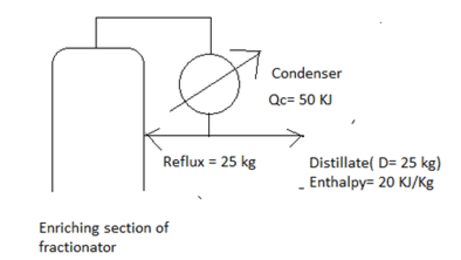
a) 20 KJ/Kg
b) 22 KJ/Kg
c) 24 KJ/Kg
d) 0
View Answer
Explanation: Heat removed by the condenser = Qc/D + Hd = 50/25 + 20 = 20 KJ/Kg.
7. Find the distillate rate by analysing the fractionator given below.
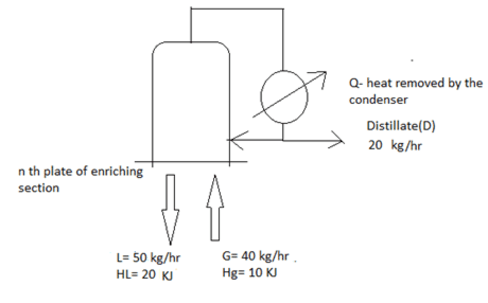
a) 10 KJ
b) 20 KJ
c) 30 KJ
d) 40 KJ
View Answer
Explanation: We have to calculate from n th tray
L*HL + G* Hg = DQ
Q=(50*20 + 40*10)/20 = 30 KJ.
8. The fictitious stream is the difference in the flow rates of the nth tray.
a) True
b) False
View Answer
Explanation: Fictitious stream = Gn+1 – Ln (Represented by delta).
9. Ponchon Savorit method do not include enthalpy concentration diagram.
a) True
b) False
View Answer
Explanation: The heat losses or heat used by the condenser or reboiler determined only by enthalpy concentration data.
10. Find the flow rate of liquid entering into the enriching section if the internal reflux ratio is 1.5 times min. Given minimum reflux ratio is 0.95 and the gas flow rate is 35 mol/hr.
a) 49.87 mol/hr
b) 22.167 mol/hr
c) 55.26 mol/hr
d) None of the mentioned
View Answer
Explanation: Internal reflux ratio = Liquid flow rate/ gas flow rate ( in nth plate of section)
1.5 *0.95 = L/35
L= 49.87 mol/hr.
Sanfoundry Global Education & Learning Series – Mass Transfer.
To practice Mass Transfer Question Bank, here is complete set of 1000+ Multiple Choice Questions and Answers
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Practice Chemical Engineering MCQs
- Apply for Mechanical Engineering Internship
- Practice Mechanical Engineering MCQs
- Check Mechanical Engineering Books
- Check Mass Transfer Books
