This set of Basic Chemical Engineering Multiple Choice Questions & Answers (MCQs) focuses on “Recycle with Chemical Reaction”.
Answer question 1 – 5 for the following diagram, the reaction is A -> 2B.
1. What is the value of W?
a) 10 mole/hr
b) 40 mole/hr
c) 50 mole/hr
d) 90 mole/hr
View answer
Explanation: W = 10 + 40 = 50 mole/hr.
2. What is the value of P?
a) 5 mole/hr
b) 10 mole/hr
c) 25 mole/hr
d) 40 mole/hr
View answer
Explanation: P = 20% of W, => P = 50*20/100 = 10 mole/hr.
3. What is the overall extent of reaction?
a) 5
b) 10
c) 20
d) 25
View answer
Explanation: Extent of reaction = (P – 0)/2 = (10 – 0)/2.
4. What is the overall fraction conversion?
a) 10%
b) 20%
c) 80%
d) 100%
View answer
Explanation: Overall fraction conversion = (10 – 0)/10*100 = 100%.
5. What is the single pass fraction conversion?
a) 10%
b) 20%
c) 25%
d) 50%
View answer
Explanation: Single pass fraction conversion = (50 – 40)/50*100 = 20%.
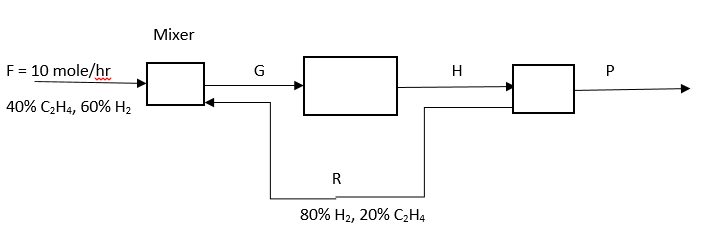
Answer the question 6 – 10 for the following diagram. The reaction is C2H4 + H2 -> C2H6, the overall conversion rate of C2H4 is 90%, and the single pass conversion rate is 20%.
6. What is the extent of reaction?
a) 3.6
b) 4.8
c) 6.4
d) 7.2
View answer
Explanation: Extent of reaction = 4*0.9/1 = 3.6.
7. What are the moles of ethane in products?
a) 2.4
b) 3.6
c) 6.4
d) 7.2
View answer
Explanation: Moles of C2H6 = 3.6*1 = 3.6.
8. What is the value of P?
a) 3.6 mole/hr
b) 5.4 mole/hr
c) 6.4 mole/hr
d) 7.8 mole/hr
View answer
Explanation: The extent of reaction = (4*0.9)/1 = 3.6, => moles of C2H6 = 3.6, moles of H2 = 6 – 3.6 = 2.4, moles of C2H4 = 4 – 3.6 = 0.4, => P = 3.6 + 2.4 + 0.4 = 6.4 moles/hr.
9. What is the value of R?
a) 18.4 mole/hr
b) 44 mole/hr
c) 70 mole/hr
d) 98.5 mole/hr
View answer
Explanation: 0.2 = – (-3.6)/(4 + 0.2*R), => R = 70 mole/hr.
10. What is the value of G?
a) 10 mole/hr
b) 30 mole/hr
c) 70 mole/hr
d) 80 mole/hr
View answer
Explanation: G = 10 + 70 = 80 mole/hr.
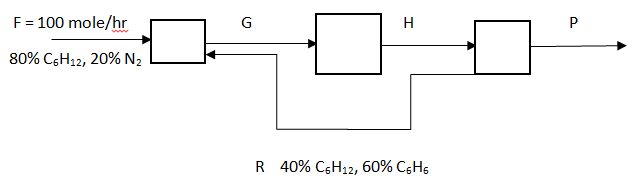
Answer question 11 – 15 for the following diagram. The reaction is C6H12 -> C6H6 + 3H2, the overall conversion of C6H12 is 80%, and single pass conversion rate is 20%.
11. What is the extent of reaction?
a) 4
b) 8
c) 12
d) 16
View answer
Explanation: Moles of C6H12 in feed = 80, => extent of reaction = (80*0.2 – 80)/(-1) = 64.
12. How many moles of benzene are formed?
a) 8
b) 16
c) 32
d) 64
View answer
Explanation: Moles of benzene formed = 64*1 = 64.
13. What is the value of P?
a) 64 mole/hr
b) 192 mole/hr
c) 272 mole/hr
d) 512 mole/hr
View answer
Explanation: Moles of benzene = 64, moles of hexane = 80 – 80*0.8 = 16, moles of H2 = 64*3 = 192, => P = 64 + 16 + 192 = 272 mole/hr.
14. What is the value of R?
a) 600 mole/hr
b) 720 mole/hr
c) 880 mole/hr
d) 960 mole/hr
View answer
Explanation: 0.2 = – (-64)/(80 + 0.4*R), => R = 600 mole/hr.
15. What is the value of G?
a) 600 mole/hr
b) 700 mole/hr
c) 800 mole/hr
d) 900 mole/hr
View answer
Explanation: G = 100 + 600 = 700 mole/hr.
Sanfoundry Global Education & Learning Series – Basic Chemical Engineering.
To practice all areas of Basic Chemical Engineering, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]